You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Carnot cycle: Definition and 108 Discussions
The Carnot cycle is a theoretical ideal thermodynamic cycle proposed by French physicist Nicolas Léonard Sadi Carnot in 1824 and expanded upon by others over the next few decades. It provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference by the application of work to the system. It is not an actual thermodynamic cycle but is a theoretical construct.
Every single thermodynamic system exists in a particular state. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. In the process of going through this cycle, the system may perform work on its surroundings, for example by moving a piston, thereby acting as a heat engine. A system undergoing a Carnot cycle is called a Carnot heat engine, although such a "perfect" engine is only a theoretical construct and cannot be built in practice. However, a microscopic Carnot heat engine has been designed and run.Essentially, there are two "heat reservoirs" forming part of the heat engine at temperatures Th and Tc (hot and cold respectively). They have such large thermal capacity that their temperatures are practically unaffected by a single cycle. Since the cycle is theoretically reversible, there is no generation of entropy during the cycle; entropy is conserved. During the cycle, an arbitrary amount of entropy ΔS is extracted from the hot reservoir, and deposited in the cold reservoir. Since there is no volume change in either reservoir, they do no work, and during the cycle, an amount of energy ThΔS is extracted from the hot reservoir and a smaller amount of energy TcΔS is deposited in the cold reservoir. The difference in the two energies (Th−Tc)ΔS is equal to the work done by the engine.
View More On Wikipedia.org
Every single thermodynamic system exists in a particular state. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. In the process of going through this cycle, the system may perform work on its surroundings, for example by moving a piston, thereby acting as a heat engine. A system undergoing a Carnot cycle is called a Carnot heat engine, although such a "perfect" engine is only a theoretical construct and cannot be built in practice. However, a microscopic Carnot heat engine has been designed and run.Essentially, there are two "heat reservoirs" forming part of the heat engine at temperatures Th and Tc (hot and cold respectively). They have such large thermal capacity that their temperatures are practically unaffected by a single cycle. Since the cycle is theoretically reversible, there is no generation of entropy during the cycle; entropy is conserved. During the cycle, an arbitrary amount of entropy ΔS is extracted from the hot reservoir, and deposited in the cold reservoir. Since there is no volume change in either reservoir, they do no work, and during the cycle, an amount of energy ThΔS is extracted from the hot reservoir and a smaller amount of energy TcΔS is deposited in the cold reservoir. The difference in the two energies (Th−Tc)ΔS is equal to the work done by the engine.
View More On Wikipedia.org
-
K
I Theoretical maximum efficiency of a heat engine without Carnot
Through an intriguing fictitious dialog between Sadi Carnot and Robert Sterling, Prof. Israel Urieli of the Ohio University shows that it is not required to invoke entropy, the second law of thermodynamics, and the Carnot cycle with the [ideal] adiabatic processes in order to find out the...- KedarMhaswade
- Thread
- Replies: 1
- Forum: Other Physics Topics
-

Engineering Carnot Cycle and Coefficient of Performance
I think I calculated part a correctly by extracting the cp (specific heat) of water from the table which is 4.188 Then calculated Q_dot by simply using the equation Q=m*c*deltaT=10.47kW But I am stuck at part b, I know that the heat extracted from the water is the same as Q_L (rate of heat...- bardia sepehrnia
- Thread
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-
A
Calculating work and heat transfer in this Carnot process
Hey guys! This is problem from Callens Thermodynamics textbook and I'm stuck with it. My goal was to get a expression for the entropy ##S## which is dependent on ##T## so I can move into the ##T-S##-plane to do my calculations: I startet by expressing the fundamental equation as a function of...- approx12
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
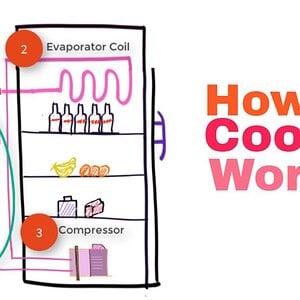
Refrigerators and Carnot Cycle (Sequence of 5 steps) #14
Refrigerators run on Carnot cycle. It is like a heat pump that operates on the reversed Carnot cycle. It utilizes the evaporation of the refrigerant to absor...- Vish
- Media item
- carnot cycle refrigerators thermodynamics
- Comments: 0
- Category: Thermodynamics
-
S
Engine working between multiple temperature baths worse than Carnot
Let the new engine, NE, extract heat from a certain subset of these baths, and let heat obtained from the ##i^{\rm th}## bath be denoted by ##Q_i##, and let the heat rejected to the ##j^{\rm th}## be denoted by ##Q_j##. Let the engine perform an amount of work ##W##. Now right beside this...- Sat D
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Conceptual thermodynamics problem about ammonia executing a Carnot cycle
Is there a mathematical explanation for why the work done in the condenser (in process 2 to 3) is zero? I am aware that ammonia does not expand or compress in the condenser, only changes phase, but without knowing that the process takes place in a condenser and only considering the graph...- Andrew1234
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Thermodynamics -- Temperature of a Heat Source?
In heat engine we define a heat source from where heat is transferred to the system, we say that heat source has a temperature ##T_h## , When we define a Carnot heat engine, the first process we have is an isothermal expansion and we say heat has to come in system through this process and here...- Rahulx084
- Thread
- Replies: 16
- Forum: Materials and Chemical Engineering
-
F
How many reversible thermodynamic cycles are there between two heat reservoirs?
Hi, I was revisiting my (high school level) understanding of thermodynamic cycles and I think I still have some doubts. Last year and more recently I posted a few questions which surely helped me, but I think I need more clarifications. In a nutshell, what I'd like to know is the following...- FranzDiCoccio
- Thread
- Replies: 12
- Forum: Thermodynamics
-
F
Estimate the mass of the water (Carnot cycle)
m=Q/c(79*ln80)- fizzyfiz
- Thread
-
- Tags
- Carnot cycle Cycle Estimate Mass Water
- Replies: 10
- Forum: Introductory Physics Homework Help
-

Efficiency of a steam power plant
My inital assumption was to just take T1 = 5600 and T2= 300K, find the maximum efficiency and then divide it by two and three but I don't believe this question to be that simple. I'm confused as to where the 750K fits in as I thought no matter what occurred in between the heat reservoir and heat...- TheBigDig
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
L
Why Do Different Methods Yield Different Efficiencies in a Reversible Cycle?
I just read about Carnot theorem (the highest efficiency is the one of reversible machines and all reversible machines working between two given temperatures have the same efficiency). Then I found a problem where I have a reversible cycle made of an isochoric, adiabatic and isotherm. I report...- L0r3n20
- Thread
-
- Tags
- Carnot Carnot cycle Cycle Theorem
- Replies: 2
- Forum: Introductory Physics Homework Help
-
F
Do Carnot and Stirling Engines Have the Same Efficiency?
Hi, I have some doubts and questions about the above thermodynamic cycles. These questions arise from some statements I find in a couple of textbooks: "Physics, 10th edition" by Cutnell et al [A], and the other is "Fundamentals of Physics" by Halliday and Resnick . Actually, I have the versions...- FranzDiCoccio
- Thread
-
- Tags
- Carnot cycle Cycle Efficiency
- Replies: 8
- Forum: Thermodynamics
-
T
Carnot Cycle efficiency, temperature dependency
Hi, We know that a heat engine working on a Carnot Cycle the efficiency is: 1 - QL/QH = 1 - TL/TH where T is in Kelvin. But if we use a different absolute temperature scale whose values at TH and TL are different, then the value of efficiency also changes. I am confused about this issue...- thinkingcap81
- Thread
- Replies: 7
- Forum: Thermodynamics
-
H
What is a good experiment for carnot cycle?
im thinking of a stirling cycle but if otherwise then please tell as soon as possible- Hrithik mudaliar
- Thread
- Replies: 2
- Forum: Thermodynamics
-

The entropy of a Carnot cycle and the efficiency equation
I have my first question. It's about entropy in the Carnot cycle and I'll try to be direct. The equal sign in the Carnot cycle efficiency equation is related to the fact that the total entropy doesn't change at the end of the whole cycle (being related to the fact that the heat exchanges occur...- Benhur
- Thread
- Replies: 5
- Forum: Thermodynamics
-
M
Calculating work done by a Carnot engine
"A Carnot engine operates using a heat source at 500 °C, and a heat sink at room temperature (20 °C). Suppose that as a heat source, you use the combustion of 100 cubic feet of natural gas at room temperature and pressure (e.g. in a fuel cell of some kind). Under ideal conditions, what is the...- Mimi Sanford
- Thread
- Replies: 14
- Forum: Introductory Physics Homework Help
-

Heat energy input from a hot source in a heat engine
Greetings! I did the famous "Mass Lifter Heat Engine" experiment in which a mass is put at the top of a piston enclosed within a cylinder. The cylinder is connected to an aluminium canister of air and the air inside this canister expands and contracts the piston with thermal contact. We had 2...- Franz Rojas Ayala
- Thread
- Replies: 11
- Forum: Thermodynamics
-
M
2 body engine, final equilibrium temperate and work produced
Homework Statement 2 bodies with contant heat capacity C and 2C, are initially at temp T and 2T. if a heat engine executing a reversible carnot cycle operates between the two bodies until their temperatures are equal, what is the final temp of the bodies and how much work is preformed by the...- mrmerchant786
- Thread
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-
J
Carnot cycle - Zero Power Extremes
Hey guys, I ran into this paper talking about the Maximum power you can obtain from a Carnot cycle: http://aapt.scitation.org/doi/abs/10.1119/1.10023 From what I understood, there are two extremes. To achieve maximum efficiency you have to make sure that the temperature of the system is never...- Joshua Pham
- Thread
- Replies: 7
- Forum: Mechanical Engineering
-
R
Carnot Cycle: Doubt in Reversible Isothermal Heat Addition
In Carnot cycle during the process - "Reversible isothermal heat addition" Q (supplied) = ∫pdV This means that the supplied heat is utilized for pdV work. My doubt is if the Q supplied is converted to work in this process then how Carnot cycle can reject heat during the upcoming isothermal...- Rahul Mohan P
- Thread
-
- Tags
- Carnot Carnot cycle Cycle Doubt
- Replies: 5
- Forum: Thermodynamics
-
P
Carnot Cycle: Analysis of Energy Exchange
A refrigerator operates on a Carnot cycle. In this cycles, it absorbs 120 J of energy at a temperature Tc while 300 J of work is done on the gas undergoing the cycle. How much energy is exhausted as heat during this process? The answer is 420 J. I am unsure of where to start for this...- physics123
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
B
Maximum work done in a Carnot Cycle
Given that in a Carnot Cycle the two adiabatic processes are essentially equal and opposite in magnitude the total work done by the cycle is in the two isotherms. The total work of the system is generally given as -NR(Th-Tc)ln(Vb/Va). Does this mean that the work done by a monatomic ideal gas is...- BrianSauce
- Thread
- Replies: 6
- Forum: Thermodynamics
-

Proving Carnot efficiency is maximum and conditions
The standard proof to show carnot efficiency cannot be exceeded is to couple a supposedly more efficient engine to a carnot refrigerator, and show that it would violate second law. However, isn't it true that we can make the same argument with any arbitrary efficiency? Some discussions on...- C_Pu
- Thread
- Replies: 1
- Forum: Thermodynamics
-
G
In 3rd step of Carnot cycle, work done on the piston is pdV?
Hello. First, look at the figure describing Carnot's cycle. In 1st step (A → B) and 2nd step (B → C), I fully understand that the work done on the pistol (surrounding) by the gas (system) is dW = pdV where dV = Adl since F = pA is the only force on the pistol from the gas (I assumed there...- goodphy
- Thread
- Replies: 18
- Forum: Thermodynamics
-

Efficient Heat Engine and Final Temperature Calculation
Homework Statement Two identical bodies of constant heat capacity ##C_p## at temperatures ##T_1## and ##T_2## respectively are used as reservoirs for a heat engine. If the bodies remain at constant pressure, show that the amount of work obtainable is ##W = C_p (T_1 + T_2 − 2T_f)##, where...- danyull
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Different states of a Carnot Cycle
Homework Statement A Carnot engine with water as the working fluid operates with a water recirculation rate of 1 kg/s. For TH = 475 K and TC = 300 K, determine: a. The pressure of each state b. The quality of each state c. The rate of heat addition d. The rate of heat...- cheme2019
- Thread
-
- Tags
- Carnot Carnot cycle Cycle States
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
A
Why is Qir < than Qr? Carnot cycle and change in G
I am learning physics on khan academy and they do a proof to show that delta G for a reversible reaction is negative and how for a irreversible reaction it is positive. However in the proof, they assume that the heat put in by the isotherm is less for an irreversible reaction compared with a...- Abhishek Jain
- Thread
- Replies: 34
- Forum: Thermodynamics
-
T
Carnot Cycle with negative temperatures
Homework Statement Heat engines at negative temperatures. Consider using two heat reservoirs to run an engine (analogous to the Carnot cycle of chapter 3), but specify that both temperatures, T_hot and T_cold, are negative temperatures. The engine is to run reversibly. (a) If the engine is to...- Thomas Brady
- Thread
- Replies: 8
- Forum: Advanced Physics Homework Help
-

How Do Carnot's Efficiency Formulas for Heat Engines Align?
My question is: according to Carnot cycle, the maximum efficiency of a heat engine is given by 1 - T2/T1, where T2 is the temperature of the cold source and T1 the temperature of the hot source. So, accordingly, as higher T2 is for a same T1, lowest is the efficiency of the engine. But, the...- Tulio Cesar
- Thread
- Replies: 1
- Forum: Thermodynamics
-

Heat Extracted and Delivered to Reservoirs in a Heat Engine
NO TEMPLATE---MISPLACED HOMEWORK So it seems like a pretty simple question, and in all likelihood it is, but my lecturer somehow managed to miss this bit in his lecture notes. A heat engine operates between 500K and 300K with 20% of the efficiency of Carnot engine operating between the same...- Willfrid Somogyi
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
M
Calculating Internal Energy Change: Path 2 of Carnot Cycle Question
Homework Statement A gas is to be expanded from initial stage i to final stage f along either path 1 or path 2 on a p-V diagram. Path 1 consists of three steps: an isothermal expansion(work is 23J in magnitude), an adiabatic expansion(work is 35J in magnitude), and another isothermal expansion...- Munir M
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
R
Carnot Cycle Use: Understand When to Apply
I saw a problem for which I don't really understand the idea of the solution. This is what it says: The vaporization latent heat for water (at 100 deg C) under normal pressure (101325 pa) is 2,3*10^6. What is the saturated vapor pressure for water at 105 deg C? And the solution says that we have...- RingNebula57
- Thread
-
- Tags
- Carnot Carnot cycle Cycle
- Replies: 1
- Forum: Thermodynamics
-
T
Learning the Carnot Cycle: 4 Steps Explained
I am learning the Carnot Cycle but I still get confused about this cycle. There are four steps in this cycle. 1)Isothermal expansion 2)Adiabatic expansion 3)Isothermal compression 4)Adiabatic compression After step one, we got the heat from heat reservoir through expansion but I don't...- Tin Yeung
- Thread
-
- Tags
- Carnot Carnot cycle Cycle
- Replies: 1
- Forum: Thermodynamics
-

Efficiency of a Carnot Engine
The efficiency of a Carnot Engine is described by the relationship: Tc/Th = Qc/Qh, so that e(Carnot) = 1 - Tc/Th For heat engines, can their efficiency also be related to temperature as well? Or is the description of their efficiency just: e(heat engine) = W / Qh = 1 - Qc/Qh I am inclined to...- vetgirl1990
- Thread
- Replies: 3
- Forum: Thermodynamics
-
A
Thermodynamics - Carnot Cycle-esque question?
Homework Statement A reversible heat engine produces work from the temperature difference that exists between a mass of m = 9 kg of an ideal gas (cv = 716 J/kgK, R = 287 J/kgK) in a rigid container and a heat reservoir at THR = 285 K. The only heat transfer interaction experienced by the... -
O
Understanding the Carnot cycle
This P-V diagram summarizes the Carnot cycle. Given a working fluid whose state ##(P,V,T)## is ##(P_1,V_1,T_1)## (at point 1) where ##PV=nRT##, the working fluid undergoes a cycle of four stages and again retrieve its original state of ##(P_1,V_1,T_1)##, that is it gets back again to point 1...- Omar Nagib
- Thread
- Replies: 1
- Forum: Thermodynamics
-
O
How possibly can heat flow between equally hot bodies?
An ideal Carnot engine is composed of two reservoirs and a working fluid. The hot Reservoir and the cold one have temperatures ##T_1## and ##T_2## respectively, with ##T_1>T_2##. The working fluid is in a phase transition and has temperature ##T_1## at the start of the Carnot cycle. It undergoes...- Omar Nagib
- Thread
- Replies: 17
- Forum: Thermodynamics
-
S
Work Done in Carnot Cycle: Adiabatic Compression
1.what is the work done in an adiabatic compression process in a carnot cyclewhen we consider work done in efficiency why do we take heat exchange into account i.e.,qs-qrThe Attempt at a Solution- s@ikiran
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Understanding the Carnot cycle
My textbook explains the Carnot cycle as follows: 1. Heat is added to the boiler, where the steam inside expands isothermally at high temperature (T(High))w(both valves closed). 2. The intake valve is opened (exhaust valve closed) and the steam expands adiabatically. The expanding steam...- Mr Davis 97
- Thread
-
- Tags
- Carnot Carnot cycle Cycle
- Replies: 3
- Forum: Thermodynamics
-

Engines in Cars: Exploring the Carnot Cycle
are engines in our cars follow the carnot cycle or they work differently- person_random_normal
- Thread
-
- Tags
- Carnot Carnot cycle Cars Cycle Engines
- Replies: 3
- Forum: Mechanical Engineering
-
Q
Volumes at C and D in a Carnot cycle
Homework Statement A Carnot cycle, shown in Fig. 20-7, has the following conditions: Va = 7.5 L, Vb = 15 L, TH = 470°C, and TL = 260°C. The gas used in the cycle is 0.50 mil of a diatomic gas, y = 1.4. Calculate (a) the pressures at a and b; (b) the volumes at c and d. (The rest of the...- Qwurty2.0
- Thread
-
- Tags
- Carnot Carnot cycle Cycle Volumes
- Replies: 3
- Forum: Introductory Physics Homework Help
-
I
How does Carnot Cycle Expand and Compress Isentropically?
Homework Statement I think I understand the first 3 steps of the Carnot cycle but not the 4th. Homework Equations The cycle here: http://en.wikipedia.org/wiki/Carnot_cycle#Stages The Attempt at a Solution I understand that in stage 1, the gas expands by taking in heat from the hot reservoir...- I<3NickTesla
- Thread
-
- Tags
- Carnot Carnot cycle Cycle
- Replies: 5
- Forum: Introductory Physics Homework Help
-
N
Reversible Carnot Cycle: Exploring Heat Dynamics & Conservation
In a reversible carnot cycle the amount of heat added is always more than the amount of heat taken out. So why doesn't it explode? I'm wondering how heat is conserved mathematically as the area under the curve of a p-v diagram. u=q+w. If the heat during expansion is always more than the heat... -
What Makes the Carnot Cycle the Most Efficient?
I have 2 questions, which are related, and was hoping someone could help me clear things up. First question, isn't the Otto cycle reversible and usable as a refrigerator? Referring to the diagram above & let me go part by part. AD: Assume the corresponding part of the working substance is...- Billy.Ljm
- Thread
-
- Tags
- Carnot Carnot cycle Cycle Otto
- Replies: 1
- Forum: Thermodynamics
-
I
Carnot cycle work & efficiency
Homework Statement The oxygen contained in a thermally insulated container will be cooled down to its boiling point (-183 C), and then condensed. Thereby one uses a reverse Carnot process operating between the oxygen gas (instantaneous) temperature and the room temperature (17 C). What is the...- Incand
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
A
Another contradiction in thermodynamics?
Consider an ideal gas operating in a quasi-static (very slow) cycle that is identical to the heat engine version of the carnot cycle in every aspect, except that friction is present. So even though the cycle is quasi-static, it is irreversible due to friction. Now the question is: How does the...- Amin2014
- Thread
- Replies: 50
- Forum: Thermodynamics
-
A
Why the Carnot Cycle? | Understanding Efficiency in Heat Engines
1-Is the efficiency of heat engines working in cycles other than Carnot independent of the nature of substance used? Can we still claim that maximum efficiency in converting heat to work is attained during reversible processes for such cycles? For which engines/cycles can we do this? 2- Why is...- Amin2014
- Thread
- Replies: 2
- Forum: Thermodynamics
-
2
Confused about the Carnot engine?
I am teaching myself thermodynamics (and really enjoying it!) but am slightly confused about Carnot's engine. From the equation efficiency=1-T(cold reservoir)/T(hot reservoir), I see that the most efficient engine is one where the difference in temperature between the cold and hot reservoirs is...- 21joanna12
- Thread
- Replies: 4
- Forum: Thermodynamics
-
R
T-S Carnot Cycle for Photon Gas
So I have been thinking about the photon gas, and I have read several papers talking about how a Carnot cycle could be created for it. This is fantastic, and it is something I am quite comfortable with. All of the papers present the P-V diagram as the "golden" Carnot cycle for the photon gas...- repetelww
- Thread
- Replies: 2
- Forum: Thermodynamics
-
T
Carnot cycle heat engine max work done
Homework Statement A heat engine containing an ideal gas has a reversible cycle which consists of 2 constant volume segments with V = 1ℓ and V = 3ℓ and two constant pressure segments with P = 1 atm and P = 2 atm (see figure below). The temperature at point “c” is Tc = 273 K. A) Is the path...- toothpaste666
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help