- #1
KedarMhaswade
- 35
- 6
- TL;DR Summary
- It seems possible to find out the theoretical maximum efficiency of a closed-circuit heat engine without invoking the Carnot cycle and its ideal adiabatic processes. Is it?
Through an intriguing fictitious dialog between Sadi Carnot and Robert Sterling, Prof. Israel Urieli of the Ohio University shows that it is not required to invoke entropy, the second law of thermodynamics, and the Carnot cycle with the [ideal] adiabatic processes in order to find out the maximum theoretical efficiency of a heat engine: https://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Carnot_Stirling/index.html
For the sake of completeness, I reproduce it here:
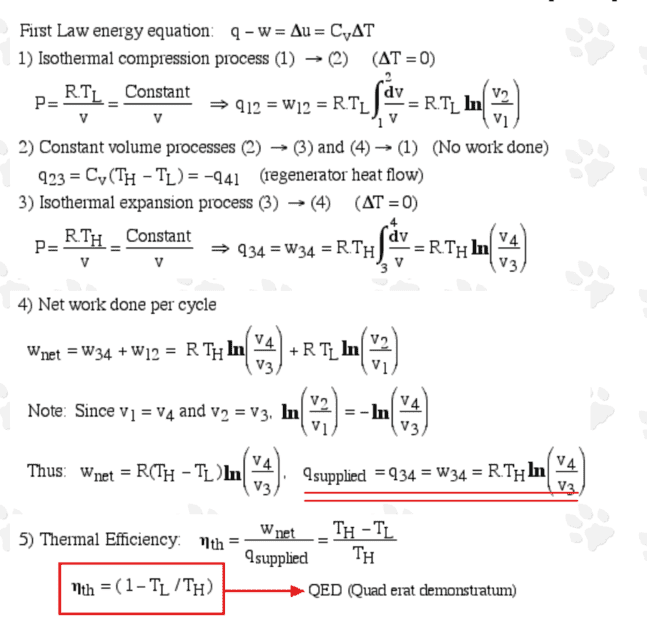
I think the analysis is plausible; the interesting thing is about the heat exchanger (regenerator) which, under ideal conditions, provides a perfect heat transfer between the gas and the regenerator equipment (including the coolant). The only slight confusion is whether ##q_{supplied} = q_{34}## is the only energy transferred into the engine by heat during the isothermal expansion?
Does anyone disagree with this analysis? Please elucidate either way. Note that apparently the professor won a prize for his http://www.centrostirling.com/isec2014/index-isec2014.html.
For the sake of completeness, I reproduce it here:
I think the analysis is plausible; the interesting thing is about the heat exchanger (regenerator) which, under ideal conditions, provides a perfect heat transfer between the gas and the regenerator equipment (including the coolant). The only slight confusion is whether ##q_{supplied} = q_{34}## is the only energy transferred into the engine by heat during the isothermal expansion?
Does anyone disagree with this analysis? Please elucidate either way. Note that apparently the professor won a prize for his http://www.centrostirling.com/isec2014/index-isec2014.html.
Last edited: