You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Wavelength: Definition and 1000 Discussions
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats. It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, troughs, or zero crossings, and is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the spatial frequency. Wavelength is commonly designated by the Greek letter lambda (λ).
The term wavelength is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids.Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to frequency of the wave: waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths.Wavelength depends on the medium (for example, vacuum, air, or water) that a wave travels through. Examples of waves are sound waves, light, water waves and periodic electrical signals in a conductor. A sound wave is a variation in air pressure, while in light and other electromagnetic radiation the strength of the electric and the magnetic field vary. Water waves are variations in the height of a body of water. In a crystal lattice vibration, atomic positions vary.
The range of wavelengths or frequencies for wave phenomena is called a spectrum. The name originated with the visible light spectrum but now can be applied to the entire electromagnetic spectrum as well as to a sound spectrum or vibration spectrum.
View More On Wikipedia.org
The term wavelength is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids.Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to frequency of the wave: waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths.Wavelength depends on the medium (for example, vacuum, air, or water) that a wave travels through. Examples of waves are sound waves, light, water waves and periodic electrical signals in a conductor. A sound wave is a variation in air pressure, while in light and other electromagnetic radiation the strength of the electric and the magnetic field vary. Water waves are variations in the height of a body of water. In a crystal lattice vibration, atomic positions vary.
The range of wavelengths or frequencies for wave phenomena is called a spectrum. The name originated with the visible light spectrum but now can be applied to the entire electromagnetic spectrum as well as to a sound spectrum or vibration spectrum.
View More On Wikipedia.org
-
S
Refraction of light and wavelength
Light of wavelength 680 nm in air enters water making an angle of 40 degrees with the normal. Find the angle of refraction and the wavelength of light in water. I found the angle of refraction, but I don't know how to calculate the wavelength with this information.- siobhanbree
- Thread
- Replies: 1
- Forum: Optics
-
C
Hydrogen Emission Spectra Wavelength
Homework Statement A transmission diffraction grating with 528 lines/mm is used to study the line spectrum of the light produced by a hydrogen discharge tube. The grating is 1.3m from the source (behind a hole in the center of a meter stick). An observer sees the first-order red line at a...- carrico
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
H
Calculating frequency and wavelength off of a graph
Homework Statement http://i213.photobucket.com/albums/cc285/havenater23/RIP.jpg?t=1301441030 I've been trying out things on this problem, I really need some help. I tried finding the frequency by counting the waves and dividing it by the seconds it takes through the waves. EX : 4 (Waves)/...- Havenater23
- Thread
-
- Tags
- Frequency Graph Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
L
Debroglie Wavelength Homework: Proton and Alpha Particle Ratio
Homework Statement The momentum of a proton and an alpha particle are equal. The mass of alpha particle is 4 times the mass of proton. The ratio of wavelength associated with them is ---- Homework Equations \lambda = h / p The Attempt at a Solution if the momentum are equal then...- logearav
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Finding the max wavelength for two waves to interfere destructively
Homework Statement i) Two waves travel between two points along paths that have the same length 4.7 x 10^-7 m, one travels in a medium having a refractive index of 3.5 while the other travels through a vacuum, how long does in take for each wave to travel between the points? ii) If the...- Sleve123
- Thread
-
- Tags
- Max Wavelength Waves
- Replies: 1
- Forum: Introductory Physics Homework Help
-
N
How do Heisenberg Uncertainty & de Broglie wavelength apply to atom trap?
I had this https://www.physicsforums.com/showthread.php?p=3200140#post3200140", which I posted on PF. I got the answer, but then I started thinking more about it and have some theoretical questions. If you did have this particle of mass m in a box of length L, which you are trying to stop...- natalie.*
- Thread
- Replies: 1
- Forum: Quantum Interpretations and Foundations
-
K
Which of the following particles would have the shortest wavelength?
which of the following particles would have shortest wavelength provided that all move with the same kinetic energy? electron, proton, neutron, alpha paricle? My professor said in class that it was alpha particle, but i think its electron... which one is right? and why?- khamaar
- Thread
-
- Tags
- Particles Wavelength
- Replies: 3
- Forum: Quantum Physics
-
C
Tensions of string of different wavelength etc
Homework Statement An A string (s1) from one musical instrument (i1), is replaced by an A string (s2) from a different instrument (i2) (both in the same family) what would be the tension ratio of string 2 after it has been replaced onto instrument 1? string 1 Length of string= 0.37 m...- Clairepie
- Thread
-
- Tags
- String Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
How Much Kinetic Energy Do Electrons Need to Resolve a 10fm Nucleus?
Homework Statement To "obeserve" small objects,one measures the diffraction of the particles whose de Broglie wavelength is approximately equal to the object's size. Find the kinetic energy(in electron volts) required by the electrons to resolve c) a nucleus of size 10fm. Homework...- blade_090
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Pair production minimal wavelength
Homework Statement what is the minimal wavelength of electromagnetic radiation to pair-produce an electron-positron pair? Homework Equations ~ = wavelength hc/~ = 2MeC^2 + K The Attempt at a Solution hc/~ = 2MeC^2 = 2(9.11x10^-31)(3x10^8)^2 ~ = 8.24x10^-11m is tis correct?- blade_090
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
W
Wavelength of a wave in a closed tube
Homework Statement In a lab that we performed, we had a closed tube with piezo electric transducers on both ends, and they were attached to a frequency generator. We found the frequencies that caused resonance, and the lab wants us to calculate the velocity of the wave using data. The lab...- warfreak131
- Thread
-
- Tags
- Closed Tube Wave Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
B
De Broglie wavelength, Maximum kinetic energy, and smoke particles
I have a few questions: 1. 'small smoke particles in air are seen under a low magnification microscope to move randomly at a speed of 0.10mm/s. The speed of sound in air is 330m/s. Estimate the mass of the smoke particles. I cannot make the link between speed of particle and speed of sound -...- Badrakhandama
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
A
Why does the angle of light through a prism change based on its wavelength?
Just a general question on the EM spectrum. angle deviation of the light wave as it passes through a prism seems to be inversely related (looks like a 1/x graph) to its wavelength. So my question, why? My guess is that the deflected path is shorter than the undeflected by cos A, where 0...- Ambidext
- Thread
-
- Tags
- Intensity Wavelength
- Replies: 1
- Forum: Other Physics Topics
-
D
Calculate Resonance Wavelength?
Calculate Resonance Wavelength?? Homework Statement Ive been asked to calculate the resonance wavelength of gas in a resonance tube. What I have to calculate this with is the frequency f and the average difference of length between each 1⁄2 wavelength measurement. It says calculate it using...- drc754
- Thread
-
- Tags
- Resonance Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Calculating Wavelength with Wave Speed and Period | Physics Homework Help
Homework Statement What is the wavelength λ of a wave that has a speed of 160 m/s and a period of 4.9 ms? Homework Equations λ = vT The Attempt at a Solution λ = 160 x 4.9 = 784m However this answer is showing up as inccorect when I submit it online. What am I doing wrong?- bfitzp
- Thread
-
- Tags
- Wavelength
- Replies: 5
- Forum: Introductory Physics Homework Help
-

De Broglie's wavelength problem
Hi, I would like a correction + help for the last question. Homework Statement A bullet of mass m=40 g travels at speed 1000 m/s. 1)What is its de Broglie wavelength? 2)Why can't we see the wavelike nature of the bullet by means of diffraction? 3)If the uncertainty of which we measured the...- fluidistic
- Thread
-
- Tags
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-

De Broglie's wavelength, what is it?
From an exercise I calculated the de Broglie's wavelength of a bullet (mass=40g and speed =1000m/s) to be very small: 1.65651 \times 10 ^{-35}m. What does this mean? That to observe some interference pattern if I throw up these kind of bullets into a double slit, I'd need a width of the slit...- fluidistic
- Thread
-
- Tags
- Wavelength
- Replies: 12
- Forum: Quantum Interpretations and Foundations
-
X
Finding the De Broglie Wavelength of a Hydrogen Atom at Room Temperature
Question: Calculate the De Broglie wavelength for a hydrogen atom at room temperature (300K). So far, the only equation I know/have used for De Broglie wavelength is lambda=h/(mv). However, I am not exactly sure how to incorporate the information that the hydrogen atom is at room temperature...- xregina12
- Thread
-
- Tags
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Find wavelength for a standing wave
Homework Statement Two sinusoidal waves with identical wavelengths and amplitudes travel in opposite directions along a string with a speed of 10 cm/s. If the time interval between instants when the string is flat is 0.50 s, what is the wavelength of the waves? Homework Equations v = w/k =...- jwxie
- Thread
-
- Tags
- Standing wave Wave Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Wavelength and destructive interference
Homework Statement In the figure the two speakers are producing identical sound waves. The solid lines represent constructive interference regions and the dashed lines represent destructive interference regions. The point labeled 1 is 662.15 m from the bottom speaker and 742.9 m from the...- boogiepicker
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Finding wavelength of sound waves
Homework Statement The two speakers are producing identical sound waves. The solid lines represent constructive interference regions and the dashed lines represent destructive interference regions. The point labeled 4 is 1228.5 m from the bottom speaker and 1618.5 m from the top speaker...- SilentBlade91
- Thread
-
- Tags
- Sound Sound waves Wavelength Waves
- Replies: 8
- Forum: Introductory Physics Homework Help
-
R
Help finding de Broglie wavelength for an electron
Homework Statement An electron in the first Bohr orbit of a hydrogen atom (a_0=5.3*10^-11m) has a KE of 13.6 eV. Express the de Broglie wavelength for this electron in multiples of the atomic circumference. Homework Equations lambda=h/p =h/(sqrt(2mqV) The Attempt at a...- reality99
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A
How Can You Measure Light Wavelengths Using Microscope Slides and Rubber Bands?
In school we somehow measured the minimum and maximum wavelength our eyes could see Unfortunately, all I remember is that we were looking through a slit between jaws of some sort of calipers and used a ruler to do measurements Can anyone please give me an insight of how to set up such...- alikim
- Thread
-
- Tags
- Light Measurement Wavelength
- Replies: 2
- Forum: Optics
-
V
Very simple problem about wavelength, just a double check on work
Homework Statement radiation with a frequency of 1200cm^-1, calculate the wavelength in meters and the frequency in hertz Homework Equations wavenumber = 1/λ freq = c/λ The Attempt at a Solution 1200cm^-1 = 1/λ λ = 8.33*10^-6 m freq = (3.0 * 10^8)/(8.66*10^-6)...- vande060
- Thread
-
- Tags
- Wavelength Work
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Calculating the 'Wavelength' Of the first harmonic
Homework Statement Hi, i have a problem that I'm trying to figure out: An open ended tube has a resonant frequency of 261.6Hz, and the speed of sound is 343m/s. What is the tubes' length?Homework Equations v=(f)(λ) v=(f)(λ/2) The Attempt at a Solution calculating with the equation v=(f)(λ)...- mHo2
- Thread
-
- Tags
- Harmonic Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
V
Wave Problem - Amp, Freq, Vel & Wave Length
Homework Statement The displacement function for a string carrying a transverse wave is y(x, t) = 2.0mm*sin(20*x/m − 600*t/s) Determine the amplitude, frequency, velocity and wavelength of the wave. Determine the maximum transverse speed of any point on the string. Homework...- vande060
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
L
Gravity Wave - Wavelength / Freq
Hi Folks Does anyone know if Gravity waves have been measured accurately enough to have a wavelength and frequency at the quantum scale. I'm looking for any data measured in Plancks. Bit of a random one, it relates to some research I am doing at the moment. Thanks Best Colin- lowing99
- Thread
-
- Tags
- Gravity Wave Wavelength
- Replies: 8
- Forum: Other Physics Topics
-
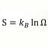
E = Em sin(kx-wt) and I = T+R what wavelength is reflected wave
When a light wave enters a medium the electric field value of the wave is smaller in the medium. With the incident wave = transmission wave + reflected wave. If the E field of the reflected wave is known . Can the wavelength of the reflected wave be obtained from these relationships : E = Em...- morrobay
- Thread
-
- Tags
- Em Wave Wavelength
- Replies: 2
- Forum: Optics
-
P
Quantum, photon, wavelength relationship
Hi Guys, Is there a SIMPLE (I am not a physicist) relationship between a quantum of energy, the photon(s) involved and the wavelength (or frequency) of the photon(s) involved in making up of the quantum? Regards. Pierre- Pierre007080
- Thread
- Replies: 25
- Forum: Quantum Physics
-
M
Finding the de Broglie wavelength from momentum
Homework Statement What is the de Broglie wavelength of a neutron traveling with a momentum equal to 300 \frac{\text{MeV}}{\text{c}}? Homework Equations \lambda = \frac{h}{p} The Attempt at a Solution p = \frac{300 \cdot \left( \left(1\times10^6 \right) \times...- Matty R
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Confused about wavelength (basic)
I'm confused about how to think about wavelength for something physical, like a simple pendulum or harmonic oscillator. I understand graphically what wavelength is but can't seem to come up with a physical description. I was thinking about ocean waves and that wavelength could be the direct...- mishima
- Thread
-
- Tags
- Confused Wavelength
- Replies: 7
- Forum: Other Physics Topics
-
D
Finding the wavelength of this wave
Homework Statement Okay, here is a photo of the wave I did on a graphing program: http://img221.imageshack.us/i/wavelengthsgraph.jpg/ Now the total distance the wave travels is 4.8 M. There are 3 crests and 3 troughs, but there is only 2 wavelengths throughout the wave. This confuses me...- -Dragoon-
- Thread
-
- Tags
- Wave Wavelength
- Replies: 6
- Forum: Introductory Physics Homework Help
-
O
Calculate wavelength of electron
Homework Statement http://www.screencast.com/users/trinhn812/folders/Jing/media/9582a1aa-b21a-4e50-8774-b60866a7d666 Homework Equations lambda=h/p The Attempt at a Solution So I got 4.04E-12 m but the book says the answer is 3.23pm I'm not sure where I'm wrong- okgo
- Thread
-
- Tags
- Electron Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
F
Wavelength of photon and size of imaged object
I have read the statement that 'the wavelength of the photons used in imaging an object needs to be much larger than the object itself', although I have never seen/heard a reason explained or the name of a theorem quoted. I have seen this in the description of more than one imaging modalities...- fred1234
- Thread
-
- Tags
- Photon Wavelength
- Replies: 7
- Forum: Quantum Physics
-
S
Work function of silver given wavelength
Homework Statement If electrons can be ejected from a silver surface using light with wavelengths as large as 262 nm. What is the work function for silver? Homework Equations work function=hf c=f\lambda The Attempt at a Solution Given the wavelength, I solved for f =...- sheepcountme
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
T
Work function of a cathode, when the wavelength of light is increased by 50%
Homework Statement The maximum kinetic energy of photoelectrons is 3.14 eV. When the wavelength of the light is increased by 50.0 %, the maximum energy decreases to 1.11 eV. What is the work function of the cathode? Homework Equations Kmax = E elec - E not = hf - hfnot hf =...- Tina20
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
U
De Broglie wavelength and mass
I've been studying quantum physics for a little over a semester now and I'm having trouble wrapping my head around the physical significance of matter waves. The derivation also confuses me because it states the momentum of particle with mass as mc, which is only true for a massless photon. I...- unchained1978
- Thread
- Replies: 2
- Forum: Quantum Interpretations and Foundations
-
R
Maximum detectable wavelength of a photoresistor
to increase maximum detectable wavelength of a photoresistor which is equal to planck's constant/ semiconductor energy gap , we need to decrease the energy gap only planck's constant cannot be increased or decreased, right?- Rula
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
A
De Broglie Wavelength - Can a Person Move Slow Enough?
Just a random thought: is it not possible for a person to move slow enough to have an observable de Broglie wavelength? For example a person moving at around 10^-30 m/s should have a wave nature that is very observable.- Arctic
- Thread
- Replies: 4
- Forum: Quantum Interpretations and Foundations
-

Radio communicattion in lower wavelength
Hi community. Once again, a stupid question: if radio waves are electromagneetic waves, generating an electric field in the antenna, then why can't one just shine visual light (also electrromagnetic waves) on same antenna and use that for communication? thanks s- Seanskahn
- Thread
-
- Tags
- Radio Wavelength
- Replies: 1
- Forum: Classical Physics
-
M
X-ray Diffraction, Intensity vs. Wavelength Graph
Homework Statement http://b.imagehost.org/0607/Question_7.png Homework Equations [PLAIN][PLAIN]http://d.imagehost.org/0813/Untitled_6.jpg The Attempt at a Solution I was able to get question b, which ends up being 73pm, but as for questions a and c, I was unable to come up with...- Mxbn0
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
F
How is the wavelength of monochromatic light measured
how is the wavelength of monochromatic light measured by using diffraction grating and spectometer?- franklinear
- Thread
-
- Tags
- Light Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
P
Finding deBroigle wavelength of a charged electron
Homework Statement Given an electron with total energy 1 KeV, determine it's deBroigle wavelength. Homework Equations E^2 = (mc^2)^2+(pc)^2 \lambda = \frac{h}{p} The Attempt at a Solution (pc)^2 = E^2 - (mc^2)^2 <> p = ± \frac{1}{c} \sqrt{E^2-(mc^2)^2} What am I doing...- ParrotPete
- Thread
-
- Tags
- Charged Electron Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
Wavelength of photon in a medium
A photon goes from vacuum into a medium. This causes its momentum to reduce. Hence by the de Broglie formula, its wavelength should increase. But, since the refractive index of the medium is greater than 1, and n_2/n_1 = \lambda_1 / \lambda_2 , and hence, its wavelength should decrease...- Muthu
- Thread
-
- Tags
- Medium Photon Wavelength
- Replies: 18
- Forum: Optics
-
N
How Do Wi-Fi and Bluetooth Devices Transmit Digital Data?
Wi-Fi Wavelength?? First of all,, HI, everybody, I am Sonics from India I was searching on Wi-Fi modems and how other bluetooth enabled or wireless things work. I know that they catch some frequency and then convert it to something that is been sent on that wavelength but I don't know that how...- niksng
- Thread
-
- Tags
- Bluetooth Wavelength
- Replies: 1
- Forum: Other Physics Topics
-
S
What is the Correct Wavelength of Light in an Interference Experiment?
Homework Statement Monochromatic light from a point source illuminates two parallel, narrow slits. The centres of the slip openings are 0.8mm apart. An interference pattern forms on a screen placed parallel to the plane of the slits and 49 cm away. The distance between two adjacent dark...- stphillips
- Thread
-
- Tags
- Light Wavelength
- Replies: 6
- Forum: Introductory Physics Homework Help
-
S
Q : Verify Wien's displacement law (frequency and wavelength form)?
HINT: SUB in (x=hv/k(b)T) I don't understand How I am supposed to approach this question or how I am supposed to verify it- sam_021
- Thread
-
- Tags
- Displacement Form Law Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
C
Wavelength and Extinction Coefficients
I have been researching on the net and books for some time now. I have a few tables and equations but was wondering if anyone has the one I am looking for? I'm looking for a table that lists Wavelength(nm), Extinction Coefficient(k), Absorption Coefficeint(a) and Density(d) values from ~300nm...- Chad Stoltzma
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
E
Math of De-Broglie wavelength boggles me
According to simple math you get \lambda=h/p for photons. and the assumption is for everything else, whilst for everything else which is NOT restmass=0 it should be \lambda=hc/E. So why is it referred to as simple \lambda=h/p when clearly it is not true mathematically. It would be true for...- etamorphmagus
- Thread
-
- Tags
- Wavelength
- Replies: 8
- Forum: Quantum Physics
-
I
Sub woofers and tweeters wavelength
[b]1. question [b]2.hello Dear , I have a question for you please , When you buy a home cinema system, you can place the subwoofer almost anywhere, even behind your couch. But the tweeters (speaker for high frequencies) must be placed very accurately. Explain why! Hint: use...- Istfan
- Thread
-
- Tags
- Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help