- #1
Janiceleong26
- 276
- 4
1. Homework Statement
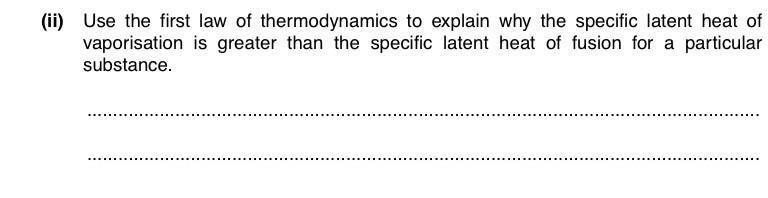
First law of thermodynamics, ΔU=q+W
Ok so, I know that when liquid evaporates, the change in volume is much greater than that when solid melts. And for both cases, distance of separation of atoms increases too, so PE increases, and hence, internal energy increases.
So, ΔU=mL+ pΔV
L=(ΔU-pΔV) /m
But how do we know that the term (ΔU-pΔV) is greater for vaporisation than that of fusion?
Homework Equations
First law of thermodynamics, ΔU=q+W
The Attempt at a Solution
Ok so, I know that when liquid evaporates, the change in volume is much greater than that when solid melts. And for both cases, distance of separation of atoms increases too, so PE increases, and hence, internal energy increases.
So, ΔU=mL+ pΔV
L=(ΔU-pΔV) /m
But how do we know that the term (ΔU-pΔV) is greater for vaporisation than that of fusion?