- #1
ikihi
- 81
- 2
Homework Statement
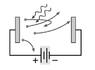
Photons with momentum p= 7.88 x 10-18 strike a photoelectric material which is in the configuration shown in the figure. The cutoff voltage is measured to be Vstop= 1.75 V. What is the work function of the photoelectric material?
Homework Equations
p = Ephoton / c
E0 = (c ⋅ p) - (Vstop ⋅ e)
The Attempt at a Solution
E0 = (3.00 x 108 m/s)(7.88 x 10-18 (ev ⋅ s)/nm) - (1.75 V ⋅ 1.602 x 10-19 J) = 2.36 eV
Attachments
Last edited: