You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Gas: Definition and 1000 Discussions
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible, as indicated by the constant velocity vectors in the image.
The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention.
High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive listing of these exotic states of matter see list of states of matter.
View More On Wikipedia.org
The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention.
High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive listing of these exotic states of matter see list of states of matter.
View More On Wikipedia.org
-

I Possible to Store Energy in Ionized Gas?
How plausible would it be to store energy in the form of ionized gas? The idea is that a gas (I am assuming a noble gas would work best) would be ionized so that some outer electrons are removed from the atoms and then a positively charged Van de Graaff generator (or something that can fulfill...- Misha Kuznetsov
- Thread
- Replies: 2
- Forum: Other Physics Topics
-

Pressure exerted by an ideal gas
Hi Please have a look on the attachment. Suppose that a 0.1 kg rubber ball having velocity of 60 m/s is moving between two walls A and B, and the distance between the walls is 1 m. It is having elastic collisions with the walls. Let's focus on what is happening at wall B. The ball is moving...- PainterGuy
- Thread
- Replies: 80
- Forum: Mechanics
-
R
Acceleration of a gas into a vacuum
Set up: Gas on one side of a divider and an infinite vacuum on the other. Question: When you remove the divider, over time, does the gas increase in acceleration, does it peak and then decrease or does it remain the same as the gas enters the vacuum/ leaves the side opposite the vacuum?f Thanks- Ron Spencer
- Thread
-
- Tags
- Acceleration Gas Vacuum
- Replies: 4
- Forum: Mechanics
-
R
Estimate average speed of electrons in gas?
Homework Statement Suppose that a heated gas comprised of electrons glows bright orange when it is in use. Estimate the average speed of the electrons in the gas. Model this gas as an ideal gas. Homework Equations None. I have no clue. This is a "review" question on a chapter titled "Current...- Rijad Hadzic
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Statistical Physics: Quantum ideal gas
Homework Statement I'm reading the book about Statistical Physics from W. Nolting, specifically the chapter about quantum gas. In the case of a classical ideal gas, we can get the state functions with the partition functions of the three ensembles (microcanonical, canonical and grand...- aburriu
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A
B Gas Behaviour in Space: Exploring the Mysteries of Gaseous Matter Beyond Earth
What happens to gases in space? Do they just dissapear? (Yes yes, yawn) Or can they make up a region of space, and stick together via gravity? And what about Jupiter and other gas giants? How do they work, if in space, all gases tend to just shoot out and spread into an even film?- Anon5000
- Thread
- Replies: 6
- Forum: Astronomy and Astrophysics
-
U
Pressure dependence of the equilibrium constant for an ideal gas
I read in some scripts that equilibrium constant for an ideal gas is not a function of pressure: But that is not generally true! Since: $$\left (\frac{\partial \Delta_{R} G}{\partial p} \right )_{T,\vec{n}}=\Delta_{R} V$$ and $$\Delta_{R} G^{0}=-RT\cdot \ln K$$ it should be: $$\left... -
B
B Internal energy of compressed gas
Hi, I've been reading about compressed air energy storage and keep coming across that in 300 bar containers the achievable energy is 0.1MJ/L. Is this 0.1MJ/L of the volume of the air it is compressed to or of the total L of air that was initially used? (E.g If 1500L is compressed to 300 bar...- Bhope69199
- Thread
- Replies: 8
- Forum: Classical Physics
-

Need a small valve for gas injection? Any ideas?
Hello everyone, For injecting gas into a little setup I've got, I have the following mechanism set out. The gas comes from a chemical reaction, goes through a copper capillary, then to a glass tube which was melted in the centre and pulled at both ends to create a thin passage. Then from that to...- eigenmax
- Thread
- Replies: 4
- Forum: General Engineering
-
K
What is the Relationship Between Gas Pressure and Fluid Column Height?
Homework Statement I need to find the amount of H2 being lost to water by being stored underneath a column of water. About 16 mL of H2 is made and it displaces the column by 16 cm. However the total amount of water is 30 ml/30 cm. I'm not sure which relationship to use to find the pressure of... -
M
How Does Box Size Affect Kinetic Energy Calculations in Gas Molecules?
In definition and proof of kinetic energy, it uses the second law of Newton. The length of the box is L and it uses this length to define the time of applying the force on the wall. Actually this time should be only time that molecule reaches to the wall and changes its velocity. My question...- majid_mahjoori
- Thread
-
- Tags
- Energy Gas Kinetic Kinetic energy
- Replies: 1
- Forum: Mechanics
-

Gas at a constant pressure: using Cv.dT correctly
Hello, my questions is not so much homework, but a request for a definition. When we use Cv*dT to solve for dU (internal energy) in a constant pressure example, what is the order of the temperatures entered into dT? Is one to assume its final temp minus initial temp? I ask because it leads to...- chopnhack
- Thread
- Replies: 10
- Forum: Introductory Physics Homework Help
-

PV Diagram of Ideal Monatomic Gas Processes
Homework Statement 1.0 mol sample of an ideal monatomic gas originally at a pressure of 1 atm undergoes a 3-step process as follows: It expands adiabatically from T1 = 588 K to T2 = 389 K It is compressed at constant pressure until its...- chopnhack
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
F
Does Rotating a Gas Container Increase Its Temperature?
If I place an insulated container of gas on the edge of a turntable and then rotate the container as a constant angular velocity, what happens to the gas? Does the temperature of the gas increase? If so, how was work done on the gas? -
C
Quick question about perfect gas
Two different gasses (Helium and Cripton) are mixed up. We can assume the compound behaves as a perfect gas only if the atoms of He and Kr have the same average value of: Mass Momentum Velocity Kinetic energy Which one is correct? I can't figure it out.- criticalPoint
- Thread
-
- Tags
- Gas Perfect gas
- Replies: 3
- Forum: Introductory Physics Homework Help
-
Z
A Gas diffusion through a semi permeable membrane
Say I have a small solid polymer container filled with gas A. The walls of the container are semi-permeable, so gas A on inside can't get through/out. On the outside, gas B at atmospheric pressure, which can migrate through the membranes of container. Pressure inside the container is 0,6 bar...- Zamw
- Thread
- Replies: 5
- Forum: Other Physics Topics
-

Internal Energy of an Ideal gas related to Molar specific heat
Homework Statement Please look at the below images which is the derivation of the relation between the internal energy of an ideal gas and the molar specific heat at constant volume. (Snaps taken from Fundamentals of Physics Textbook by David Halliday, Jearl Walker, and Robert Resnick) As...- SciencyBoi
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Gas under pressure- disturbing noise recurring
recently my neighbors and I have been awakened by a loud noise that sounds like something is being released under high pressure. An intense vibrating sound that lasts 30-45 seconds. We live across the steet from a soda manufacturer and down the road from a natural Gas transfer station. Both are...- Pat Bannan
- Thread
- Replies: 2
- Forum: Chemistry
-
H
How to find added thermal heat in monoatomic gas?
Homework Statement for number 3,4,5 I'm still tryingHomework Equations PV/T = PV/T q = ΔU + W W = P ΔV The Attempt at a Solution (3) I used PV/T = PV/T to find the ΔT for each process for A→B I find PV/TA = P3V/TB ----- TB = 3TA (T increase) for B→C I find P3V/TB = 4P3V/TC ----- TC =...- Helly123
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
P
Ideal Gas Law -- Isobaric Epansion followed by....
Homework Statement An ideal gas with Cv = 5/2R, and γ = 1.4 starts at a volume of 1.5m3 , a pressure of 2.0×105Pa, and a temperature of 300K. It undergoes an isobaric expansion until the volume is V , then undergoes an adiabatic expansion until the volume is 6.0m3 , and finally undergoes an...- physics123
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-

Final velocity of an air rifle pellet from compressed gas
As in the title, I'm trying to establish the approximate velocity (sans friction and other losses) of a pellet propelled by compressed gas from a tank. Below is what I have came up with myself, I would appreciate if someone could review this as say whether the end values are reasonable. I have... -
F
What caused the change in a pilot light at my workplace?
This has been bugging me for a few months now, and I'm hoping that someone can provide me with an answer. A while ago, my colleague was soldering with an oxy-acetylene torch and paused for a few moments in between jobs, leaving only the pilot light on top of the unit burning. Sitting about 4-5...- fleetingmoment
- Thread
- Replies: 29
- Forum: Optics
-
B
Equation of states for a gas that forms dimers
Homework Statement Show that to a first approximation the equation of state of a gas that dimerizes to a small extent is given by, ##\dfrac{PV}{RT} = 1 - \dfrac{K_c}{V}## Where ##K_c## is equilibrium constant for ##A + A \iff A_2## Homework EquationsThe Attempt at a Solution Using virial...- Buffu
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
A
How mass flow and pressure ratio is related in case of a Gas Turbine
Hi, As per my observation and experience, when we use evaporative cooler to cool the inlet air to the gas turbine, the compressor discharge pressure (i.e PCD or P3) rises. As, the temp. of the inlet air drops and the mass flow increases due to the increase in density, I believe the discharge...- Ady707
- Thread
- Replies: 5
- Forum: Aerospace Engineering
-

Liquid to gas expansion temperature change in a tank
Hello ! I have troube doing a calculation. Let's say we have a volume Vi in a tank at a pressure Pi. If I let my tank open, the tank's liquid will start to boil to keep the pressure Pi inside the tank. Now, at the end, I will have a volume Vf in the tank, and still a pressure Pi. But the...- PHstud
- Thread
- Replies: 3
- Forum: Thermodynamics
-
G
What is the power of a car engine?
Homework Statement The volume displacement in a car engine is 2000 cm3. During the power stroke, the mean pressure inside the cylinder is 15 bar. Compute the work performed by the engine in one revolution. Compute the engine's power assuming it runs at 3000 rpm. Homework Equations...- greypilgrim
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-

New Concept for Zero Emission Natural Gas Power Generation
Science mag news article on a new electrical power plant being set-up by a start-up. It burns natural gas with pure oxygen and runs turbines with CO2. It makes water rather than using it and is in theory close to competitive economically. . Sounds good to me, but I'm no engineer.- BillTre
- Thread
- Replies: 3
- Forum: General Engineering
-
B
Compressing Gas: Work Done Calculation
Homework Statement [/B] A closed cylinder with a piston is used to compress gas that is initially at 1bar and temperature 293K. The compression is performed adiabatically until the volume is 1m3 and the pressure is 10bar, calculate the work done. Homework Equations [/B] pV = nR0T - ideal gas...- bassguitar
- Thread
-
- Tags
- Compression Gas
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
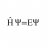
Ideal gas pressure from Maxwell-Boltzmann distribution
Hi everyone I'm having trouble with solving an exercise in statistical physics. I need to argue why the average number of particles with a velocity between ##v## and ##v+dv## that hit a surface area ##A## on the container wall in a time interval ##\Delta t## is $$N_{collision}=v_{x}A\Delta t...- H Psi equal E Psi
- Thread
- Replies: 4
- Forum: Electromagnetism
-
M
Mass Flow Rate of Flue Gas in Sugar Industry
Hello, I want to find the mass flow rate of flue gas, i have only the diameter of pipe and ID fan speed which are 1.48Meter and 980 RPM respectively- Mahesh H D
- Thread
- Replies: 2
- Forum: Mechanical Engineering
-

Change of internal energy of an ideal gas
Homework Statement Homework Equations Δu = ∫ [(a-Ru)+bT+cT^2+dT^3]dT The Attempt at a Solution The answer of 6447kJ/kmol is given but I am struggling to get to this answer after integrating the above formula and inserting the given values. Firstly would the integral of...- DevonZA
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
J
Trying to build a gas collecting chamber above a packed bed
Hi guys, I'm a graduate student and currently doing some research on a packed bed - I want to collect gas data from the headspace of my samples so i thought of building a gas collecting chamber on top of the vessel, where i can take gas measurements using calorimetric gas tubes. I'm not sure...- jen0leb
- Thread
- Replies: 1
- Forum: General Engineering
-
A
About filled gas inside a Geiger Muller meter
Hi, I have a dumb question about the filled gas inside a Geiger Muller meter. I know it is considered as a gas detector. It requires a filled and quench gas for the probe. My question is: where does the gas get injected into for like a pancake probe. (Let's use Ludlum Model: 44-9 as example)...- aznsaiyan1029
- Thread
- Replies: 2
- Forum: Nuclear Engineering
-
R
Partition function for ideal gas for "medium" temperature
Looking for the heat capacity of ideal gas due to rotational degrees of freedom. If the temperature of the gas is much higher than the temperature corresponding to the energy differential between states,the partition function can be written as the integral over the density of states. If the...- rockyleg
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

What's inside a gas detection Station?
Good Morning guys! I'm trying to understand more about gas sensors. I know a lot about electrochemical and metaloxide sensors. I've faced all the limits that those sensors presents when you are using them outdoors. Now I'm wondering how the gas sensors stations can measure different gases...- ervays
- Thread
- Replies: 4
- Forum: General Engineering
-

What is the Work Done by a Gas in a Cycle?
Homework Statement Homework Equations PV = nRT \\ W = - \int_{V_i}^{V_f} P dV \Delta E_{int} = Q + W The Attempt at a Solution a)[/B] Since this is a cyclic process, the change in internal energy of the system is 0. \Delta E_{int} = 0 The process causes some ice to melt, meaning heat...- knc
- Thread
- Replies: 24
- Forum: Introductory Physics Homework Help
-

Automotive Exhaust Gas Pressure/Blowdown Calculations
Hi! I am a bit confuzzled by these equations given by a highly referenced and cited paper I have been using to create a spreadsheet I have been working on. The equations are: PV=mRT Where P is the cylinder pressure, m is the mass of gasses in the cylinder, R universal gas constant of the gas...- Jason Louison
- Thread
-
- Tags
- Calculations Exhaust Gas
- Replies: 30
- Forum: Mechanical Engineering
-
F
Theory behind gas tubes in lab experiments
For chemical experiments that involve gases as reactant and products, gas tube are used for their transport. Q1. Why are there differences in length of gas tube when in a round flask. In most case, entering tubes are longer than exiting tubes? Why is that? Q2. Why is sometimes the entering... -
P
Calculating gas pressure at a changing system
Hello, I'm having a problem calculating the final pressure before condenser. I'm calculating the pressure that builds up in a system that at the beginning has atmospheric pressure @ T=20C and is introduced with 1 mole/second of CO2(44.01g/mol) @ T=60C and 0.318 mole/second of CH2Cl2(84,93 g/mol)...- Povilas
- Thread
- Replies: 9
- Forum: Materials and Chemical Engineering
-
G
Cooling a gas by releasing it into vacuum: Entropy?
Hi. I just read an article where following cooling method is described. Apparently it's very common, but I don't know what it's called: A gas under pressure is released into a vacuum through a small hole. The average particle speed in this beam of gas is the same as before, but the...- greypilgrim
- Thread
-
- Tags
- cooling entropy gas temperature vacuum
- Replies: 5
- Forum: Thermodynamics
-
S
Why does a gas behave as ideal if the Isotherm and Isenthelp
Homework Statement Why does a gas behave as ideal if the Isotherm and Isenthalp are parallel. Homework Equations h=u+pv T1=T2, P1V1=P2V2 The Attempt at a Solution [/B] h1=u1+p1v1, h2=u2+p2v2 h2=h1 so u1+p1v1=u2+p2v2 and p1v1=p2v2 Does that mean internal energy for an ideal gas is zero? I...- ScareCrow271828
- Thread
-
- Tags
- Gas
- Replies: 3
- Forum: Introductory Physics Homework Help
-
W
Perception of Intl Journal of Greenhouse Gas Control - Impact Factor 4
The international Journal of Greenhouse Gas Control. It has an Impact Factor of around 4, but what's the perception? Is it respected? Is it considered a good journal? There isn't a qualitative judgment online. My boss seems to look down on it, but it has a good impact factor.- womfalcs3
- Thread
- Replies: 2
- Forum: General Discussion
-
E
Ideal gas in a cylinder with a piston
Homework Statement A vertical cylinder of radius r contains an ideal gas and is fitted with a piston of mass m that is free to move. The piston and the walls of the cylinder are frictionless, and the entire cylinder is placed in a constant-temperature bath. The outside air pressure is p0. In...- Elias Waranoi
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-

B Why doesn't dark matter reside in gas clouds?
Why is it that dark matter does not inhabit gas clouds, I would have thought that thees gas clouds will some day become a galaxy, so why no Dark Matter?- wolram
- Thread
-
- Tags
- Dark matter Gas Matter
- Replies: 4
- Forum: Cosmology
-
R
How does pressure ratio and intercooling affect gas turbine performance?
Hey Mech Eng Forum, I'm currently examining the performance on gas turbine and wondering: How is the performance of gas turbines (Primarily thermal efficiency and net-work output) influenced by the pressure ratio and temperature constraints? Additionally, what is the benefit of 2 stage...- roberto t
- Thread
- Replies: 2
- Forum: Mechanical Engineering
-

A Classical gas with general dispersion relation
i'm trying to understand the solution to this problem: http://physweb.bgu.ac.il/COURSES/StatMechCohen/ExercisesPool/EXERCISES/ex_2065_sol_Y13.pdf (link to the problem and the solution of it) All my questions come from the partition function: 1) From where the term (2*pi)^d comes from?, I...- victor94
- Thread
- Replies: 1
- Forum: Classical Physics
-
A
I Time of cooling to equilibrium in space plasma gas
Hi all, If I have a hot object in space (not a star but say an oven or just a hot gas as would be on Earth < 10,000 degrees Kelvin) glowing at a temperature T and I want to know long it takes to come to equilibrium with the vacuum of space around it, how can I calculate such a time? I could...- Albertgauss
- Thread
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-
C
Calculating Entropy Change for an Ideal Gas Expansion
MENTOR NOTE: NO TEMPLATE BECAUSE SUMITTED TO WRONG FORUM. 3.1) A quantity of 0.10 mol of an ideal gas A initially at 22.2 degrees C is expanded from 0.200 dm3 to 2.42 dm3 . Calculate the values of work (w), heat (q), internal energy change (delta U), entropy change of the system (deltaSsys)...- chemochem
- Thread
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
R
Thermodynamics Gas Mixture - Dew Point Temperature
Homework Statement Only Number 1, not number 2 Homework Equations Dew Point Temperature T = Saturated Temperated at Vapor Pressure Partial Pressure = (mole fraction) x (Mixture Pressure) The Attempt at a Solution The dew point temperature is only dependent on the pressure of the water...- Reefy
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
R
Gas Mixture - Dew Point Temperature
Homework Statement Only Number 1, not number 2 Homework Equations Dew Point Temperature T = Saturated Temperated at Vapor Pressure Partial Pressure = (mole fraction) x (Mixture Pressure) The Attempt at a Solution The dew point temperature is only dependent on the pressure of the water...- Reefy
- Thread
-
- Tags
- Dew point Gas Mixture Point Temperature
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help