You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Hydrogen: Definition and 1000 Discussions
Hydrogen is the chemical element with the symbol H and atomic number 1. With a standard atomic weight of 1.008, hydrogen is the lightest element in the periodic table. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all baryonic mass. Non-remnant stars are mainly composed of hydrogen in the plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons.
The universal emergence of atomic hydrogen first occurred during the recombination epoch (Big Bang). At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most nonmetallic elements, most of the hydrogen on Earth exists in molecular forms such as water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions because most acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production is mainly from steam reforming natural gas, and less often from more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is problematic in metallurgy because it can embrittle many metals, complicating the design of pipelines and storage tanks.
View More On Wikipedia.org
The universal emergence of atomic hydrogen first occurred during the recombination epoch (Big Bang). At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most nonmetallic elements, most of the hydrogen on Earth exists in molecular forms such as water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions because most acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production is mainly from steam reforming natural gas, and less often from more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is problematic in metallurgy because it can embrittle many metals, complicating the design of pipelines and storage tanks.
View More On Wikipedia.org
-
M
Why does helium have a greater first ionization energy than hydrogen?
I have had this question in the back of my mind for a while. Hydrogen has 1 proton and 1 electron, and the atom is electrically neutral. So that means that the electrical charge from the electron and the proton cancel each other out as they have equal/opposite charge. Then when you add another... -
T
Number of electron of a Hydrogen atom (molecule)
Hi, If the normalized 1D wave-function of hydrogen atom for n=1, l=0, m_l=0; \psi_{1s}(x)=\frac{1}{\sqrt{\pi} a_{0}^{3/2}}e^{-x/a_{0}} and probability distribution of wave-function, \mid\psi_{1s}(x)^2\mid so integration of rho over all x should give the number of electrons which is equal to...- torehan
- Thread
- Replies: 8
- Forum: Quantum Physics
-
M
Hydrogen Atom Tables: Find Zeros of Spherical Bessel Function
I've searched google for some decent tables but couldn't find any. I'm trying to collect Normalized Spherical Harmonics Associated Legendre Polynomials Zeros of the Spherical Bessel Function Normalized Radius Function for the Hydrogen atom etc. I'm allowed a sheet with as much of these...- Matthollyw00d
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Fusion of the isotopes of hydrogen
Hi I assume that two hydrogen atoms fuse together to form one helium atom during nuclear fusion. But what happens when other isotopes of hydrogen fuse together? What happens to two deuterium atoms when they fuse together? Tritium? I'm assuming that they would form an isotope of helium, but... -
S
Can anyone confirm the ground state Bohr radius for muonic hydrogen?
Thanks in advance for anybody who is kind enough to help me. No this isn't for my homework. I am not even enrolled in school. I am doing some calculations for personal research. But I need to know the ground state radius of a muonic hydrogen atom to help prove my theory. I already know the...- seattle.truth
- Thread
- Replies: 1
- Forum: Quantum Physics
-
P
Matrix element of Position Operator For Hydrogen Atom
Find <nlm|(1/R)|nlm> for the hydrogen atom (nlm = 211), R is just the radial position Operator (X2 + Y2 + Z2)½.- physics2004
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
G
Solving the Dirac Equation for the Hydrogen Atom
Would someone tell me some website where I can find the relativistic treatment of the hydrogen atom using Dirac's Equation? I am not trying the find the method which uses Schrodinger's equation and adds as perturbations fine and hyperfine structures? Thank you. So far i have not find anything...- go quantum!
- Thread
- Replies: 2
- Forum: Quantum Physics
-
M
What are the inconsistencies and errors in common measurement units?
i know the rules of increasing and decreasing atom sizes down and across the periodic table. however, i have met one strange thing when i was given data to plot a graph. the hydrogen atom radius was stated as 32 pm, but helium was 50 pm. when i searched the internet, i found few websites... -
A
The Hydrogen Atom (Schrodinger vs original Bohr theory)
What quantities appear in both the Schrodinger approach and the original Bohr theory of a hydrogen atom? Also, in what ways do the two approaches differ? I'm not sure which quantities appear in both approaches. My guess would be that it has something to do with the energy levels and angular...- aheizler
- Thread
-
- Tags
- Atom Bohr Hydrogen Hydrogen atom Theory
- Replies: 1
- Forum: Quantum Physics
-
M
Lowest Energy State of Hydrogen Atom: Find A, Don't Miss the Obvious!
A hydrogen atom is in the state \psi=Ar^2e^{-r/a}cos(\theta). I need to find lowest energy state and etc. Obviously normalize to find A, but I'm not seeing the obvious linear combination of wave functions; and I really don't think my instructor wants me to do several inner products (plus...- Matthollyw00d
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom State
- Replies: 5
- Forum: Advanced Physics Homework Help
-
S
Can Hydrogen be made from air and water?
Is forming ammonium nitrate and hydrogen from air and water is possible. N2+3H2O > NH4NO3 +H2 Is this reaction exothermic? Ammonium nitrate has enthalpy of formation of -365.1- sr241
- Thread
- Replies: 9
- Forum: Materials and Chemical Engineering
-
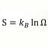
Newtons Law of Cooling applied to hot Neon and Hydrogen gas
Homework Statement 1 mole of Hydrogen gas at 300 Kevin is contained in a thin walled copper container. In container #2 there is 1 mole of Neon gas also at 300 Kevin. The volume= 22.4 liters and surface area ,A = .476 m^2 The surroundings are at 100 deg Kelvin. The specific heat of H2= 5... -
D
Solving the Mystery of Hydrogen Atom Ionization
Here comes a pretty hard question, which not even my QM teacher has been able to answer. When we think about one hydrogen atom, and put it in an electric field along the z-axis \bar E = \bar e_z E. Then the potential for a hydrogen atom will look like this: U = -\frac{e^2}{4\pi \epsilon_0...- dreamspy
- Thread
- Replies: 10
- Forum: Advanced Physics Homework Help
-
H
Proving the Visible Emissions of Balmer Series in Hydrogen Atom
Hello, I don't know if this is the right place to post.. this is a self study question from Levine, it is told that is important but i have no clue in solving it .. The Balmer series corresponds to a set of emissions involving the electron in a hydrogen atom relaxing from a high energy...- heavenchemist
- Thread
- Replies: 6
- Forum: Advanced Physics Homework Help
-
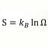
Cooling rates for Hydrogen and Neon gas
Homework Statement 1 mole of Hydrogen gas at 300 Kelvin is in a thin walled copper container vol 22.4 liters surface area A = .476 M^2 In vessel #2 , 1 mole of Neon gas also 300 Kevin The specific heat of H2= 5 cal/mole (K) Thermal conductivity (k) H2 = .0433 cal/sec. converts to .00866... -
L
What characteristic of a base enables it to ''accept'' Hydrogen Ions?
According to the Brønsted-Lowry definition, a base is a compound that can -keyword- accept H+, and an acid anything that can give H+.- LogicalAcid
- Thread
-
- Tags
- Base Characteristic Hydrogen Ions
- Replies: 2
- Forum: Chemistry
-
C
About the unit of Radial wave function R(r) of Hydrogen atom
The 1s radial function of the wave function of H atom is: R10=2 a-3/2e-r/a ,where a = 5.29*10-11 meter but substituting a with its value,we will get R10 = 5.2*1015 *e(-1.89036*1010 r) and that is impossible if r=a and R(r)=1.9*1015 where is the problem ? What's more, the unit...- caoyuan9642
- Thread
- Replies: 5
- Forum: Quantum Physics
-
C
Is an Electron a Boson or a Fermion?
I'm studying particle physics now and am confused with all these terminologies.- Chaste
- Thread
-
- Tags
- Atom Fermion Hydrogen Hydrogen atom
- Replies: 6
- Forum: Quantum Physics
-
P
Muonic hydrogen ground state energy
Would the energy just be a multiple of how much bigger it is than electron?- petey_hb69
- Thread
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
B
First-order time-dependent perturbation theory on a Hydrogen atom
Homework Statement A Hydrogen atom is initially in its ground state and then subject to a pulsed electric field E(t)=E_{0}\delta(t) along the z direction. We neglect all fine-structure and hyperfine-structure corrections. Homework Equations 1. It is important to use selection rules to avoid...- boyu
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
Allowed Transition from 2s^2 to 2p^2 in Hydrogen: Why is it Missing?
Why is there no allowed transition from the 2s^2 S_{\frac{1}{2}} state to the 2p^2 P_{\frac{3}{2}} state in the attached image? It seems to fulfill the selection rules \Delta l = \pm 1 and \Delta j = 0, \pm 1. This is for electric dipole transitions by the way.- barnflakes
- Thread
-
- Tags
- Hydrogen Rules Selection rules
- Replies: 1
- Forum: Atomic and Condensed Matter
-
U
Calculate Transition in Hydrogen for 600nm Wavelength
Homework Statement use the RYDBERG formula to suggest a possible transition that would result in the observed wavelength Homework Equations Hydrogen - yellow wavelength about 600nm The Attempt at a Solution I used c= lambda x frequency(V) and found (V) to equal 5e14/second I then...- unf0r5ak3n
- Thread
-
- Tags
- Hydrogen Transition
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
M
Add carbon atom to pure hydrogen
Looking for ideas that allow me to create Methane ( CH4 ) from gaseous hydrogen. This requires bonding one carbon atom to four hydrogen atoms, which should be simple enough, but there needs to be a minimum explosive danger. I know there are appliances to go the other way, but don't have the... -
G
Solving Hydrogen Wavefunctions with Coulomb Potential
Hello I have a question if someone could help I would be grateful. We have a wave function \psi(r,\theta,\varphi) = R_{5,4}Y_{l,m} + R_{5,3}Y_{3,0} + R_{5,2}Y_{2,1} which is describing a hydrogen atom. I have to find out for what values of the orbital quantum number and magnetic quantum...- gosuly
- Thread
-
- Tags
- Hydrogen Wavefunctions
- Replies: 1
- Forum: Advanced Physics Homework Help
-
N
Calculating Rotational & Vibrational Energy of Hydrogen Molecule
Homework Statement The potential energy V between the two hydrogen atoms ( mH = 1.672*10^(-24) g) in a hydrogen molecule is given by the empirical expression V = V0 [ exp(-2a(r-r0)) - 2exp(-a(r-r0))] where V0 = 7*10^(-12) erg, a = 2*10^8 cm-1, r0 = 8*10^-9 cm. (a) Estimate the...- nicky04
- Thread
-
- Tags
- Energy Hydrogen Molecule Rotational
- Replies: 6
- Forum: Advanced Physics Homework Help
-
M
Ionizing solutions to the hydrogen atom
Are there any ionizing solutions for the hydrogen atom problem, where the electron breaks away from the proton?- mordechai9
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom
- Replies: 5
- Forum: Quantum Physics
-
S
Negative oxygen ions reaction with hydrogen
Under what conditions and how will negative oxygen ions react with hydrogen and hydrocarbons (such as methane or gasoline)?Does it require some catalyst or should be burned together? What is a way to harness spare electrons from oxygen when water is formed? -
C
Time-averaged potential of hydrogen
Homework Statement The time-averaged potential of a neutral hydrogen atom is given by V = \frac{q}{4 \pi \epsilon_0} \frac{e^{-\alpha r}}{r} \left ( 1 + \frac{\alpha r}{2} \right ) [tex]\alpha = 2/a_0[/itex], where a_0 is the Bohr radius. Find the charge distribution (continuous and...- cscott
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
T
Genius | Calculating Hydrogen Needs for a Constantly Flying Blimp
Hi. How much hydrogen dose it take to keep a blimp in flight. Let’s say I want 2000 kg of lift, and to keep it flying constantly. How much hydrogen would I need per day or hour? If I had a steel cable with a reinforced tube to keep hydrogen pumping into the blimp from the ground, what amounts...- threadmark
- Thread
-
- Tags
- Hydrogen
- Replies: 18
- Forum: Materials and Chemical Engineering
-
K
General calculation of the oscillation freq of a hydrogen molecule
Homework Statement Okay here's the problem, normally I can get all this stuff, but right now this is blowing my mind, partly because its too general. "In other problems and examples in the textbook we found the effective spring stiffness corresponding to the interatomic force for aluminum...- Kibbel
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
K
Sodium Percarbonate as I want to use it as Hydrogen Peroxide
Hi chaps I wonder if anyone can help - I'm really stuck! I have purchased Sodium Percarbonate as I want to use it as Hydrogen Peroxide. I want to use it at 6% strength but do not know what ratio to mix it with water with! The data sheet is here... -
N
About mixed integral for perturbation in hydrogen atom
Homework Statement During my calculation of hydrogen atom perturbation, I need to integral below in cartesian coordinate. It is given that below integral can be transformed. Homework Equations Anyone could help to see what will the transformed integral in polar coordinate if the...- ntusg
- Thread
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
M
What the heck is ionized hydrogen?
My professor just attempted to explain ionized hydrogen to us. She said that an ionized hydrogen atom is basically a proton. But I don't understand why that would still be considered hydrogen if it no longer has an electron. -
L
Potential/Kinetic/Total Energy of hydrogen atom absorbing photon
Homework Statement A hydrogen atom in the n = 2 state absorbs a photon with wavelength 380 nm. Q1. Find the change in the atom’s total energy. Q2. Find the change in the atom’s potential energy Q3. Find the change in the orbiting electron’s kinetic energy. Homework Equations...- louza8
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
W
What is the Probability of Finding an Electron in a Specific Angle in Hydrogen?
Homework Statement Calculate the probability of finding the electron in a hydrogen within the angle \pm30\circ from the x-y plane.The hydrogen is in the (2,1,1) state. Homework Equations probability = \int\int\int\left|R_{2,1,1}\right|^{2} \left|Y^{1}_{1}\right|^{2} r^{2} sin(\theta) dr...- wavingerwin
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
A
Angular momentum of hydrogen atom in specific energy level
Homework Statement A hydrogen atom is in a state with energy -0.278 eV. Homework Equations L = nh' The Attempt at a Solution The answer book says to do E_n = (-13.6 eV)/n^2 but I can't find this equation in our book. Is the 13.6 a significant number or just a different number...- accountkiller
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
C
How many hydrogen atoms are there in a 5 sample of pure hydrogen?
How many hydrogen atoms are there in a 5 sample of pure hydrogen?- convict4703
- Thread
- Replies: 4
- Forum: Mechanics
-
R
Hydrogen atom, muon substitution, helium muon fusion
Homework Statement Substitute an electron in a neutral hydrogen atom with a muon. a) calculate the Bohr radius of the ground state for this myonic atom of atom. The answer must be right to at least 2 significant digits. b) Calculate the fraction of the myon that is located inside the proton...- rayman123
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
R
Hydrogen atom, muon substitution
Homework Statement Substitute an electron in a neutral hydrogen atom with a muon. a) calculate the Bohr radius of the ground state for this myonic atom of atom. The answer must be right to at least 2 significant digits. b) Calculate the fraction of the myon that is located inside the proton...- rayman123
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
X
Hydrogen vs Atomic: Nuclear Fission vs Fusion Explained | Damage Comparison
Homework Statement What is the difference between nuclear fission and nuclear fusion? After answering the question proceed on to answer the next question Between Hydrogen Bomb and Atomic Bomb, which bomb causes more damage. provide you answer with reasons. Homework Equations Nuclear...- xujf1996
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
C
Expected r^2 for the 2s wavefunction of hydrogen atom
Homework Statement Calculate the expected value for r^2 for the 2s wavefunction of the hydrogen atom (only the radial part of the function is needed for l=0). If you choose to solve this problem graphically, plot or sketch the function you integrate. Homework Equations...- chall
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
V
The Double Helix - Hydrogen bonding and stability
Rank the following base pairs according to their stability. Rank from most to least stable. To rank items as equivalent, overlap them. ------------- I have found out that the first one is thymine-adenine pair and the second one is a cytosine-guanine pair. The third one is cytosine...- Vivianian
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
S
Can Intercalation of Hydrogen Ions Solve the Problem of Hydrogen Storage?
There exist such interesting issue as intercalation, which seems to allow, for example, to store 4.4 intercalated Li ions per one (!) Silicon atom.The latest is a host material.I think if there will be found a material which would allow to store the same ratio of intercalated hydrogen ions...- Stanley514
- Thread
- Replies: 4
- Forum: Chemistry
-
O
Why is hydrogen the most common element in the universe?
i understand that everything else is less as they are (in turn) made from hydrogen, but why was so much of hydrogen made in the big bang? i got a feeling because one proton and one electron is stable. (similarly, helium was produced as 2 nucleons + 2 electrons is also very stable) but I've... -
H
Renewable hydrogen electrolysis system, advice needed
Hello I'm currently in the middle of a dissertation project, a feasibility study of a tidal/hydrogen generation system. At the moment I'm getting pretty bogged down trying to model the electrolyser. I'd like it to be more detailed than simply power in*conversion efficiencies. In particular...- hypervalve
- Thread
- Replies: 2
- Forum: General Engineering
-
Z
How much pressure can be created by the decomposition of Hydrogen peroxide?
We are a simple robotics group working in Antarctica. We have a small but nimble ROV that we launch through a hole we drill in the ice. We need to lift heavy objects off the sea floor. I want to use the decomposition of H2O2 to initiate a state change that creates the buoyancy to lift these... -
Z
What is the meaning of the electron energy states in a hydrogen atom?
You see hydrogen electron energy states labeled as 1s1/2, 2s1/2, 2p1/2 and 2p3/2. My confussion may be with understanding the definitions, but does not the 2s1/2 state imply that there are 2 electrons around as the 1st s orbital is filled. But, by definition doesn't that mean you are dealing...- zincshow
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom
- Replies: 8
- Forum: Quantum Physics