- #1
MrBi11
- 11
- 0
Greetings,
A Thesis regarding the historical accuracy of portrayal Sadi Carnot and Lord Kelvin's work seeks a University.
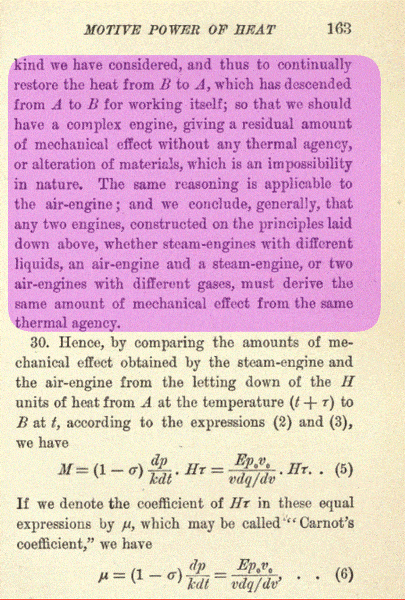
It is based largely on the images below (note highlighted areas), taken from their original work.
As you know, Sadi Carnot and Lord Kelvin are the fathers of the science of thermodynamics.
The moderator has cautioned me not to express my opinion on this forum, as he considered it not mainstream.
I presume the fathers of modern science are suitably mainstream.
References: Carnot, Sadi "REFLECTIONS ON THE MOTIVE POWER OF HEAT AND ON MACHINES FITTED TO DEVELOP THAT POWER", and Thompson, Sir William (Lord Kelvin) "AN ACCOUNT OF CARNOT'S THEORY", New York: Wiley and Sons and London: Chapman & Hall, Limited, 1897.
Thanks to www.archive.org for the scan of original print copy of "REFLECTIONS ON THE MOTIVE POWER OF HEAT".




A Thesis regarding the historical accuracy of portrayal Sadi Carnot and Lord Kelvin's work seeks a University.
It is based largely on the images below (note highlighted areas), taken from their original work.
As you know, Sadi Carnot and Lord Kelvin are the fathers of the science of thermodynamics.
The moderator has cautioned me not to express my opinion on this forum, as he considered it not mainstream.
I presume the fathers of modern science are suitably mainstream.
References: Carnot, Sadi "REFLECTIONS ON THE MOTIVE POWER OF HEAT AND ON MACHINES FITTED TO DEVELOP THAT POWER", and Thompson, Sir William (Lord Kelvin) "AN ACCOUNT OF CARNOT'S THEORY", New York: Wiley and Sons and London: Chapman & Hall, Limited, 1897.
Thanks to www.archive.org for the scan of original print copy of "REFLECTIONS ON THE MOTIVE POWER OF HEAT".