- #1
PhysicsDuki
- 1
- 0
- Homework Statement
- A bottle filled with nitrogen under a pressure of 1.5x10^7 Pa at a temperature of 27 ° C has a mass of 97 kg. Part of the nitrogen is consumed, so the pressure in the bottle, at a temperature of -3 ° C, is 6x10^6 Pa, and the weight of the bottle is 93.5 kg. How much mass of nitrogen is left in the bottle?
- Relevant Equations
- pxV = m/M x R x T
(R = 8.314 J/K x Mol and M= Molar mass of N2 ( 28 g/mol) )
Solution from the textbook:
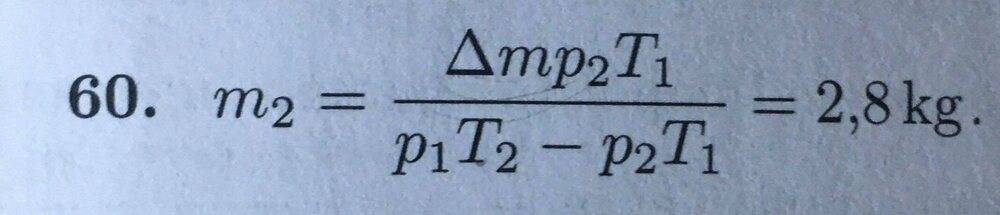 My work:
My work:

I constantly get 1.55kg. I also tried dividing the first and the second equation (pxV=m/M x R x T with different values). How did they come up with the equation in the solution? Also, I am sorry if I posted it in the wrong place and didn't follow the rules of the forum, but I am new member. Thanks in advance!
I constantly get 1.55kg. I also tried dividing the first and the second equation (pxV=m/M x R x T with different values). How did they come up with the equation in the solution? Also, I am sorry if I posted it in the wrong place and didn't follow the rules of the forum, but I am new member. Thanks in advance!