- #1
Hak
- 709
- 56
- Homework Statement
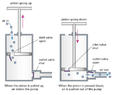
- A bicycle wheel pump works as shown in the figure below. When you pull up on the pump knob, the cylindrical barrel of the pump fills with air from the outside, which enters through a one-way valve. When the knob is pushed down, the air compresses into the tube until its pressure reaches that of the inner tube connected to the pump. At that point, another one-way valve allows air to go from the pump into the inner tube.
Assume that any compression is isothermal, as if you push the knob very slowly, so that the temperature remains in equilibrium with the external environment. Assume also that there is no air leakage from either the pump or the inner tube.
The volume of a pump barrel is [tex]\displaystyle V_P = 0.3[/tex] liters; the pump is connected to an air chamber of volume [tex]\displaystyle V_C = 4.5[/tex] liters. Assume that the air chamber is inflexible, so its volume does not increase significantly while inflating it. Initially, the pressure in the air chamber equals the atmospheric pressure [tex]\displaystyle p_A = 101.3[/tex] kPa.
1.) Using the pump, push the knob down and then up 4 times, so you transfer air 4 times from pump to inner tube. After you do this, what is the pressure in the inner tube?
2.) You might imagine that you have a pump 4 times the size of the one you have, with which you would only have to press down on the knob once to fill the inner tube. If [tex]\displaystyle W_1[/tex] is the work done to fill the inner tube with four compressions of the small pump and [tex]\displaystyle W_2[/tex] that of the air pump, how much extra work is worth required in the first case, [tex]\displaystyle W_2 - W_1[/tex]?
- Relevant Equations
- /
The pressure in the inner tube after four transfers of air from the pump is given by the formula:
[tex]\displaystyle p_C = p_A \left(\frac{V_C + V_P}{V_C}\right)^n[/tex]
where [tex]\displaystyle n[/tex] is the number of transfers, [tex]\displaystyle p_C[/tex] is the pressure in the inner tube, [tex]\displaystyle p_A[/tex] is the atmospheric pressure, [tex]\displaystyle V_C[/tex] is the volume of the inner tube, and [tex]\displaystyle V_P[/tex] is the volume of the pump.
Plugging in the given values, we get:
[tex]\displaystyle p_C = 101.3 \left(\frac{4.5 + 0.3}{4.5}\right)^4[/tex]
[tex]\displaystyle p_C = 101.3 \times 1.0667^4[/tex]
[tex]\displaystyle p_C = 101.3 \times 1.2978[/tex]
[tex]\displaystyle p_C = 131.4 kPa[/tex]
Therefore, the pressure in the inner tube after four transfers of air from the pump is 131.4 kPa.
2.) The work done by the pump to transfer air from the pump to the inner tube is given by the formula:
[tex]\displaystyle W = nRT \ln\left(\frac{V_C + V_P}{V_C}\right)[/tex]
where [tex]\displaystyle n[/tex] is the number of moles of air, [tex]\displaystyle R[/tex] is the gas constant, [tex]\displaystyle T[/tex] is the temperature, and [tex]\displaystyle V_C[/tex] and [tex]\displaystyle V_P[/tex] are the volumes of the inner tube and the pump, respectively
For the small pump, we have:
[tex]\displaystyle W_1 = nRT \ln\left(\frac{V_C + V_P}{V_C}\right)[/tex]
For the large pump, we have:
[tex]\displaystyle W_2 = nRT \ln\left(\frac{V_C + 4V_P}{V_C}\right)[/tex]
The difference between these two works is:
[tex]\displaystyle W_2 - W_1 = nRT \left[\ln\left(\frac{V_C + 4V_P}{V_C}\right) - \ln\left(\frac{V_C + V_P}{V_C}\right)\right][/tex]
Using the property of logarithms, we can simplify this expression as:
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{V_C + 4V_P}{V_C + V_P}\right)[/tex].
Plugging in the given values, we get:
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{4.5 + 4 \times 0.3}{4.5 + 0.3}\right)[/tex]
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{5.7}{4.8}\right)[/tex]
[tex]\displaystyle W_2 - W_1 = nRT \times 0.1698[/tex]
Therefore, the extra work required in using the large pump should be proportional to ##nRT \times 0.1698##.
I am not convinced by this process; I think it is wrong. Could you tell me where I am wrong? Do you have any suggestions or help? I had thought of a method where you could use the polytropic index of compression, setting it equal to 1 in the case of isothermal compression, but I can't physically formalize it.
[tex]\displaystyle p_C = p_A \left(\frac{V_C + V_P}{V_C}\right)^n[/tex]
where [tex]\displaystyle n[/tex] is the number of transfers, [tex]\displaystyle p_C[/tex] is the pressure in the inner tube, [tex]\displaystyle p_A[/tex] is the atmospheric pressure, [tex]\displaystyle V_C[/tex] is the volume of the inner tube, and [tex]\displaystyle V_P[/tex] is the volume of the pump.
Plugging in the given values, we get:
[tex]\displaystyle p_C = 101.3 \left(\frac{4.5 + 0.3}{4.5}\right)^4[/tex]
[tex]\displaystyle p_C = 101.3 \times 1.0667^4[/tex]
[tex]\displaystyle p_C = 101.3 \times 1.2978[/tex]
[tex]\displaystyle p_C = 131.4 kPa[/tex]
Therefore, the pressure in the inner tube after four transfers of air from the pump is 131.4 kPa.
2.) The work done by the pump to transfer air from the pump to the inner tube is given by the formula:
[tex]\displaystyle W = nRT \ln\left(\frac{V_C + V_P}{V_C}\right)[/tex]
where [tex]\displaystyle n[/tex] is the number of moles of air, [tex]\displaystyle R[/tex] is the gas constant, [tex]\displaystyle T[/tex] is the temperature, and [tex]\displaystyle V_C[/tex] and [tex]\displaystyle V_P[/tex] are the volumes of the inner tube and the pump, respectively
For the small pump, we have:
[tex]\displaystyle W_1 = nRT \ln\left(\frac{V_C + V_P}{V_C}\right)[/tex]
For the large pump, we have:
[tex]\displaystyle W_2 = nRT \ln\left(\frac{V_C + 4V_P}{V_C}\right)[/tex]
The difference between these two works is:
[tex]\displaystyle W_2 - W_1 = nRT \left[\ln\left(\frac{V_C + 4V_P}{V_C}\right) - \ln\left(\frac{V_C + V_P}{V_C}\right)\right][/tex]
Using the property of logarithms, we can simplify this expression as:
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{V_C + 4V_P}{V_C + V_P}\right)[/tex].
Plugging in the given values, we get:
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{4.5 + 4 \times 0.3}{4.5 + 0.3}\right)[/tex]
[tex]\displaystyle W_2 - W_1 = nRT \ln\left(\frac{5.7}{4.8}\right)[/tex]
[tex]\displaystyle W_2 - W_1 = nRT \times 0.1698[/tex]
Therefore, the extra work required in using the large pump should be proportional to ##nRT \times 0.1698##.
I am not convinced by this process; I think it is wrong. Could you tell me where I am wrong? Do you have any suggestions or help? I had thought of a method where you could use the polytropic index of compression, setting it equal to 1 in the case of isothermal compression, but I can't physically formalize it.