- #1
armageddon9007
- 1
- 0
I understand how a galvanic element works at an electrochemical level. I understand how the electrons move from the anode to the canode, and also how electrolysis works.
I do not understand how to relate the following in an electrochemical perspective:
Battery that drives charge through a circuit
If we consider the analogy which is being applied here:
The battery is considered as lifting a charge from a position of low potential energy to a place with high potential energy. A more exact explanation may be the following:
http://www.physicsclassroom.com/Class/circuits/u9l1b.cfm#circuits
On the bottom of the page you can find the explanation beneath "Electric Potential in Circuits"
Again, I understand this in a general way, but not how this way of thinking relates to the microscopic or electrochemical perspective. If we now consider the battery in the circuit in the clip (link above) as a galvanic element of zink and copper:
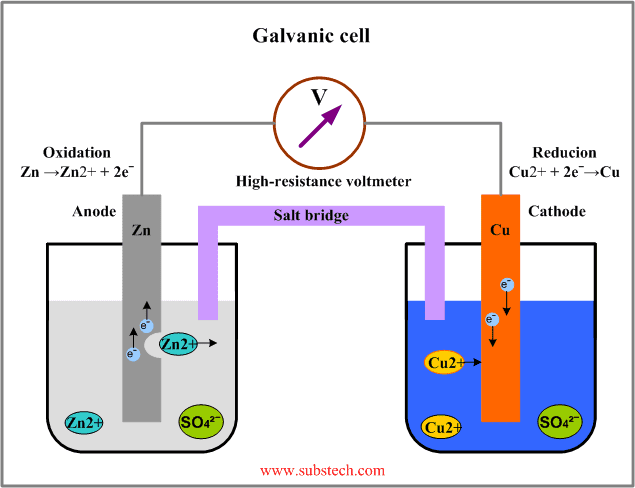
Where does this "lifting" within the battery enter the picture? According to the explanation above it is as if the battery lifts charge from a side with low potential energy (which I assume is the equivalent of the cathode) to a place with high potential energy (anode), but in a galvanic element the charge flows just one way from the anode to the cathode. I understand how a galvanic element pushes charge from a place of high potential energy to a place with low potential energy, but not the other way around. If charge is to flow the other way an electrolysis must be performed, in which the battery is charged. But according tot he explanation above it is as if the battery, when it works as a basic galvanic element, lifts charge from a place with low potential energy to a place with high potential energy by pushing it against the electric field, without performing an electrolysis. This I do not understand, and I would like an explanation.
Charging a capacitor:
If the battery in this video is a galvanic element () then I do not understand how charge flows "through" the galvanic element at an electrochemical level. Do the electrons go to the negative side because the battery says there has to be a potential difference, thus the electrons go to the oppposite side because there should be more electrons on the other side?
I do not understand how to relate the following in an electrochemical perspective:
Battery that drives charge through a circuit
If we consider the analogy which is being applied here:
The battery is considered as lifting a charge from a position of low potential energy to a place with high potential energy. A more exact explanation may be the following:
http://www.physicsclassroom.com/Class/circuits/u9l1b.cfm#circuits
On the bottom of the page you can find the explanation beneath "Electric Potential in Circuits"
Again, I understand this in a general way, but not how this way of thinking relates to the microscopic or electrochemical perspective. If we now consider the battery in the circuit in the clip (link above) as a galvanic element of zink and copper:
Where does this "lifting" within the battery enter the picture? According to the explanation above it is as if the battery lifts charge from a side with low potential energy (which I assume is the equivalent of the cathode) to a place with high potential energy (anode), but in a galvanic element the charge flows just one way from the anode to the cathode. I understand how a galvanic element pushes charge from a place of high potential energy to a place with low potential energy, but not the other way around. If charge is to flow the other way an electrolysis must be performed, in which the battery is charged. But according tot he explanation above it is as if the battery, when it works as a basic galvanic element, lifts charge from a place with low potential energy to a place with high potential energy by pushing it against the electric field, without performing an electrolysis. This I do not understand, and I would like an explanation.
Charging a capacitor:
If the battery in this video is a galvanic element () then I do not understand how charge flows "through" the galvanic element at an electrochemical level. Do the electrons go to the negative side because the battery says there has to be a potential difference, thus the electrons go to the oppposite side because there should be more electrons on the other side?
Last edited by a moderator: