- #1
Matthew Adams
- 1
- 0
Hey there folks!
This is my first post so please be gentle... ;-)
One of my students has been studying the emission spectrum of an acetylene flame. A gas mixture from both an acetylene cannister & an oxygen canister is fed out to a nozzle, a flame is ignited and we study the emission spectrum...
For a minimum of oxygen, we get the following spectrum:
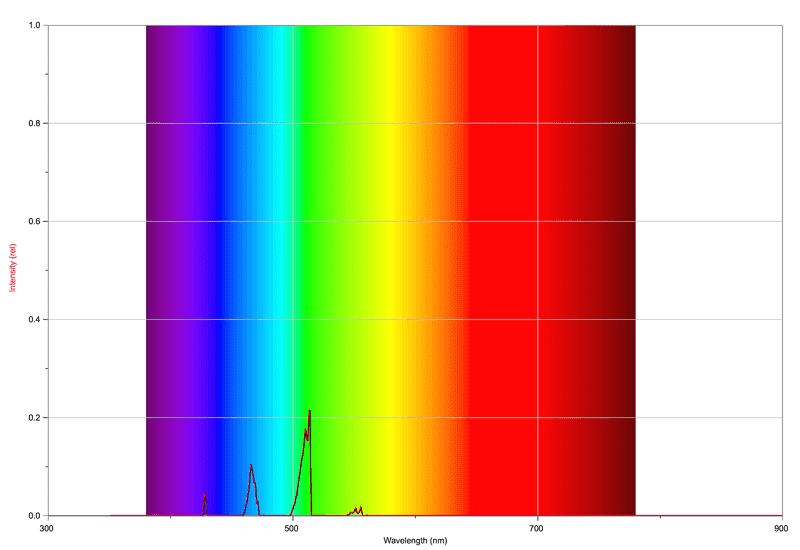
So far, so good. The peaks are as far as I understand from CH & C2 in the combustion of acetylene. The flame has a nice blue colour to it.
The fun begins when we start to increase the amount of oxygen in the fuel mix:
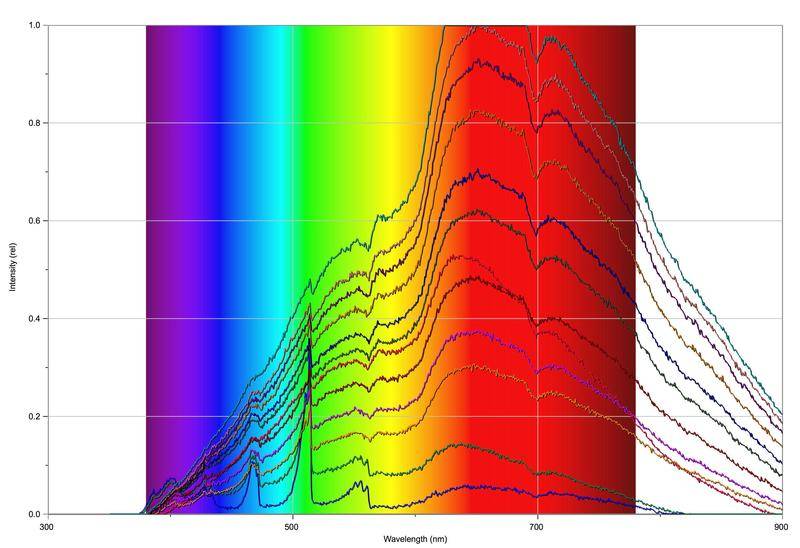
So basically, the more oxygen that is let in, the higher the measured intensity and the more the spectrum takes on the form of a black body spectrum. Around about 610-620 nm there's a slight dip and around 700 nm there's a very prominent dip that grows more significant the more oxygen that's released into the mix. As you might expect, the flame also grows more orange/yellow and more sooty, the more oxygen that's fed into the mix.
So my question is basically: what's going on? Not too sure about the dip around 610-620nm, but I think the dip at 700nm is from O2 (that or water).
If anyone can shine some light on this and give some explanation for why the intensity increases the way it does for increased oxygen, what's causing those dips and why the spectrum takes on the form of BB radiation for increasing oxygen content in the fuel, I'd be more than grateful.
This is my first post so please be gentle... ;-)
One of my students has been studying the emission spectrum of an acetylene flame. A gas mixture from both an acetylene cannister & an oxygen canister is fed out to a nozzle, a flame is ignited and we study the emission spectrum...
For a minimum of oxygen, we get the following spectrum:
So far, so good. The peaks are as far as I understand from CH & C2 in the combustion of acetylene. The flame has a nice blue colour to it.
The fun begins when we start to increase the amount of oxygen in the fuel mix:
So basically, the more oxygen that is let in, the higher the measured intensity and the more the spectrum takes on the form of a black body spectrum. Around about 610-620 nm there's a slight dip and around 700 nm there's a very prominent dip that grows more significant the more oxygen that's released into the mix. As you might expect, the flame also grows more orange/yellow and more sooty, the more oxygen that's fed into the mix.
So my question is basically: what's going on? Not too sure about the dip around 610-620nm, but I think the dip at 700nm is from O2 (that or water).
If anyone can shine some light on this and give some explanation for why the intensity increases the way it does for increased oxygen, what's causing those dips and why the spectrum takes on the form of BB radiation for increasing oxygen content in the fuel, I'd be more than grateful.