- #1
Guest432
- 48
- 2
I've been reading up on electron diffraction for electron microscopy, and I have been trying to understand the proof for the wavelength of an electron in a tunneling electron microscope. The proof I have been trying to emulate begins as follows:
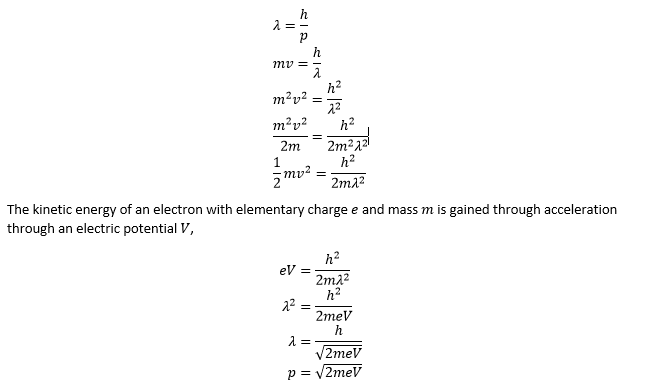
It then says that I must account for relativistic effects so, ##E^2=p^2c^2+m^2c^4## and manages to yield this term for momentum
How did it jump from ##p=\sqrt {2meV}## to that? (Note delta ##\Delta E = eV##)
It then says that I must account for relativistic effects so, ##E^2=p^2c^2+m^2c^4## and manages to yield this term for momentum
How did it jump from ##p=\sqrt {2meV}## to that? (Note delta ##\Delta E = eV##)
Last edited: