You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Oxygen: Definition and 374 Discussions
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. After hydrogen and helium, oxygen is the third-most abundant element in the universe by mass. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O2. Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Dioxygen provides the energy released in combustion and aerobic cellular respiration, and many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as do the major constituent inorganic compounds of animal shells, teeth, and bone. Most of the mass of living organisms is oxygen as a component of water, the major constituent of lifeforms. Oxygen is continuously replenished in Earth's atmosphere by photosynthesis, which uses the energy of sunlight to produce oxygen from water and carbon dioxide. Oxygen is too chemically reactive to remain a free element in air without being continuously replenished by the photosynthetic action of living organisms. Another form (allotrope) of oxygen, ozone (O3), strongly absorbs ultraviolet UVB radiation and the high-altitude ozone layer helps protect the biosphere from ultraviolet radiation. However, ozone present at the surface is a byproduct of smog and thus a pollutant.
Oxygen was isolated by Michael Sendivogius before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774. Priority is often given for Priestley because his work was published first. Priestley, however, called oxygen "dephlogisticated air", and did not recognize it as a chemical element. The name oxygen was coined in 1777 by Antoine Lavoisier, who first recognized oxygen as a chemical element and correctly characterized the role it plays in combustion.
Common uses of oxygen include production of steel, plastics and textiles, brazing, welding and cutting of steels and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight and diving.
View More On Wikipedia.org
Oxygen was isolated by Michael Sendivogius before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774. Priority is often given for Priestley because his work was published first. Priestley, however, called oxygen "dephlogisticated air", and did not recognize it as a chemical element. The name oxygen was coined in 1777 by Antoine Lavoisier, who first recognized oxygen as a chemical element and correctly characterized the role it plays in combustion.
Common uses of oxygen include production of steel, plastics and textiles, brazing, welding and cutting of steels and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight and diving.
View More On Wikipedia.org
-
E
Oxygen Absorber at 200-350 deg F For Curing Photopolymer
Basic Question: I am trying to heat cure a photopolymer for a couple of hours at 350 deg F in an anoxic atmosphere. My idea was to seal the part in aluminum foil with charcoal to absorb the oxygen, but was not sure that the carbon and oxygen would react at that temperature. Extra Background...- EricM81
- Thread
-
- Tags
- Oxygen
- Replies: 3
- Forum: Other Physics Topics
-

Why Does O₂ & O₃ Stay in the Stratosphere?
we all know that the stratosphere has ozone O₃ molecules and also O₂.:.but O₂ and O₃ are heavy molecules (comparitively) why are they all the way up there(>10km) shouldn't they come down? My guess ... because of that altitude maybe the centrifugal force acting on the molecules is enough to keep...- Suraj M
- Thread
-
- Tags
- Oxygen
- Replies: 2
- Forum: Other Physics Topics
-

Oxygen and mercury in a tube problem
Homework Statement We have a tube with the top end open and the bottom end closed. There is some oxygen gas in the tube and on top of the oxygen there is 10 cm high column of mercury. The initial temperature is 20oC. Then the tube is flipped over and heated to 40oC. The column of mercury shifts... -
D
Calculating Oxidation State of Nitrogen in 2NO + O2 -> 2NO2
2NO + O2 -> 2NO2 "Oxidation of NO" So a reactant - 2NO doesn't seem to lose an electron, it only gains an additional oxygen atom. So, I guess, oxidation is also gain of an oxygen? ... -
J
What happens when you remove an excessive amount of electrons.
Lets take a simple water molecule for example. You can use the photoelectric effect to remove electrons from a molecule. As a thought experiment, let's say you have a contained single water molecule in a vacuum with no impurities. Perfect Vacuum. If you use the Photoelectric effect and beam...- Jason White
- Thread
- Replies: 8
- Forum: Atomic and Condensed Matter
-

Oxygen Enriched Internal Steam Compression Ignition Engine (OEISCIE)
Hello PF members and guests, I have been working on this concept for 2 years and I wanted to get some input from this forum. I have done a lot of research on oxygen enriched combustion and water injection. Both of these concepts have been tested and proven in experiments and the real world. My...- voltech444
- Thread
- Replies: 14
- Forum: Mechanical Engineering
-
D
Find the Oxygen Mass in a Welding Tank
Homework Statement A welder using a tank of volume 7.40×10−2m3 fills it with oxygen (with a molar mass of 32.0g/mol ) at a gauge pressure of 3.20×105Pa and temperature of 38.7∘C. The tank has a small leak, and in time some of the oxygen leaks out. On a day when the temperature is 22.9∘C, the... -
V
Behavior of gaseous oxygen in a closed box under magnetic field?
Hi all, I'm new here, and I hope I'm doing this right. I mean posting where I should. Feel free to let me know. ;-) First, I'm a biologist but I'm a scientist and I'm interested in magnets recently. I read that magnetic field attract oxygen liquid (all over you tube) and also gaseous since...- Vander
- Thread
- Replies: 2
- Forum: Electromagnetism
-
S
Force of Flowing Gaseous Oxygen
Hi guys, I am trying to calculate the force that gaseous oxygen would exert if it were to flow from a tube and come to a restriction in that tube. I'm not sure if I am correct in my thinking, but so far, I think that the flow can be calculated by the size and length of the restriction, and then...- Seaningtime
- Thread
- Replies: 1
- Forum: Other Physics Topics
-

O2 Absorption Capacity Varies w/ Temperature: Alkyl Pyrogallol Solution
I am looking for a liquid whose oxygen absorption capacity varies with temperature. I have heard of alkyl pyrogallol solution but there are different solutions of pyrogallol in alkaline solution . I wanted to produce oxygen with the help of this o2 absorbing capacity variation with temperature. -
S
Calculate equilibrium dissolved oxygen at 19.8 Celsius
Homework Statement 1. Calculate the equilibrium dissolved oxygen concentration for each temperature if PO2 = 21% (this means that if atmospheric pressure is 1 atm then O2 pressure is 21% of that) Homework Equations K19.8/RT = Kp PV=nRT The Attempt at a Solution K19.8/RT = Kp K19.8 =...- spooky01
- Thread
-
- Tags
- celsius Equilibrium Oxygen
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
D
Other Reactions That Place When Hydrogen and Oxygen are combined
After reading this article, Water Vapor Found on Neptune-size Alien Planet - http://www.space.com/27251-water-found-neptune-size-exoplanet.html, I was wondering what other reactions would occur within the atmosphere of the planet as a result of the combination of oxygen at 25% to the 90% volume... -
S
How much oxygen absorbing area does an insect have?
Homework Statement Insects don't need lungs. They breath through their skins. In this way they can get enough oxygen to fuel the basic metabolism that is common to all life. Approximate an insect by a cylinder of diameter 4mm and length 5mm. Assuming that the density of the insect is...- salmayoussef
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
F
Diffusion rate of oxygen through skin
Homework Statement i) At rest a 70 kg person with an area of 1.7 m2 consumes around 14 litres of oxygen per hour. Around 2% of this is provided by diffusion through their skin. Calculate the diffusion rate for oxygen through human skin, in millilitres per second per square metre. ii) What... -
S
Oxygen toxicity and Hyperbaric Chambers
First post here guys. Very quick background which brings me to the question i have. My trade background is in the automotive industry as a mechanic, mines as a drill fitter, hobbies include flying, cars and scuba diving. Just recently i have changed career paths again, and will be...- stoney85
- Thread
- Replies: 4
- Forum: Biology and Medical
-
S
How long could you survive without oxygen after running?
Suppose you exerted yourself quite hard and ran for a while, then you were immediately deprived of oxygen. How long could you actually survive compared to deprived of oxygen at rest?- Soliptic
- Thread
- Replies: 1
- Forum: General Discussion
-
C
Oxygen atmosphere without ozone layer
OK, so I would like the following idea: -planet which already had its Great Oxygenation Event and life forms including human beings would be able to breath there freely -no oxygen layer or any other plausible mechanism which would practically preclude any daily life forms on the surface (all...- Czcibor
- Thread
-
- Tags
- Atmosphere Oxygen Ozone
- Replies: 7
- Forum: Sci-Fi Writing and World Building
-
Q
PH dependence of reduction of oxygen
Homework Statement Why is this true? Why is it that the reduction of oxygen is more favored in acidic solution rather than in basic solution? Homework Equations Acids are proton donors. The Attempt at a Solution Does this have anything to do with the fact that the...- Qube
- Thread
- Replies: 15
- Forum: Biology and Chemistry Homework Help
-
B
Medical Medical Grade Plastics for Oxygen Delivery
Hi everyone, I have a question about regulations for medical materials. I want to know if there are any sort of restrictions on what kinds of plastics and or other materials you can use for oxygen delivery. A bit of background on the question. My grandmother is in a nursing home and...- Bobalony
- Thread
- Replies: 4
- Forum: Biology and Medical
-
J
Angular velocity of an oxygen molecule.
Homework Statement The atoms in the oxygen molecule [O][/2] may be considered to be point masses separated by a distance of 1.2 x 10^-10 m. The molecular speed of an oxygen molecule at s.t.p. is 460 m/s. Given that the rotational kinetic energy of the molecule is two-thirds the of its...- j_namtirach
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
A
Obtain Oxygen: Break Apart CO2 or H2SO4?
Is it possible, and if so how, to obtain oxygen by breaking apart Carbon Dioxide or Sulphuric Acid?- Anon Imous
- Thread
-
- Tags
- Oxygen
- Replies: 6
- Forum: Chemistry
-
C
Searching for Non-Corrosive Material Against Water & Oxygen
Hello gentlemen - New to the forum here! ... I have what I hope is an easy question for my Materials & Chemical buds. Is there any material that is 100 percent non-corrosive against water and oxygen (sometimes nitrogen)? ... I work for a Gas Analytical company, whereas we test the...- Cadman5150
- Thread
- Replies: 2
- Forum: Materials and Chemical Engineering
-

Carbon dioxide & oxygen balance
Green Plants do Photosynthesis in day during presence of sunlight and intake C02 to produce O2. In night, Plants do respiration and intake 02 to produce C02. If there will be no animal then Green plants will survive ? Because Earth is like ball, Plants will do photosynthesis=respiration...- Infinite/Zero
- Thread
- Replies: 2
- Forum: Biology and Medical
-

Measuring Oxygen Content of the Ancient Earth
I read that the level of oxygen in the atmosphere reached its highest concentration during the Carboniferous period, with over 32% of the atmosphere by volume as O2. I was wondering, how is this measured? The reference on wiki only links to a graph with no other content. Thanks.- Drakkith
- Thread
- Replies: 2
- Forum: Earth Sciences
-

Sigma and Pi Bonds for Diatomic Oxygen Molecule
According to the Molecular orbital theory, diatomic oxygen should have σ2px (internuclear axis) and \pi2py and \pi2pz orbitals filled with two unpaired electrons, one at antibonding \pi2py and the other at antibonding \pi2pz. And of course, the 2s bonding and antibonding orbitals as well... -
I
Underwater oxygen extraction device
Hello everyone. I'm sure there is a really good reason as to why this hasn't been done yet but I just wanted to ask and see if it could be done. Why can't we make a mouth piece that basically takes in water, filters out the oxygen and then expels the water back into the ocean, then takes in...- iDimension
- Thread
-
- Tags
- Device Extraction Oxygen Underwater
- Replies: 5
- Forum: General Engineering
-
T
What is the flame temperature for burning alkali metals in oxygen
what is the flame temperature for burning alkali metals in oxygen- taregg
- Thread
-
- Tags
- Metals Oxygen Temperature
- Replies: 2
- Forum: Thermodynamics
-
G
Force on a pure oxygen bubble in air by a magnet
There are the famous experiments, which show, how an Oxygen soap bubble gets attracted by a magnet due to it's paramagnetism. And how a Nitrogen bubble gets repelled by it (in air)But how does one calculate the attraction force of this pure Oxygen soap bubble in air due to a magnet. I'm Sorry... -
P
Oxygen Phase Diagram: Pressure & Liquefy 0-50 atm
i need to know what pressure oxygen will liquefy at under various pressures but i can't find a phase diagram anywhere any link phase diagram that shows pressures 0-50 atm would be useful- pixelpuffin
- Thread
-
- Tags
- Diagram Oxygen Phase Phase diagram
- Replies: 4
- Forum: Other Physics Topics
-
N
Mixture of hydrogen and oxygen in container
Homework Statement In a closed container with a volume of V = 5 50 l is a mixture of hydrogen and oxygen with the total mass m = 50 g. The container have pressure p = 300 000 Pa and the temperature t_1 = 20 ° C. A) What is the number of moles n_1 (hydrogen) and n_2 (oxygen) in the container...- Numeriprimi
- Thread
- Replies: 27
- Forum: Introductory Physics Homework Help
-
J
Introduction of Oxygen and Hydrogen
Does introducing small amount of hydrogen and oxygen with gasoline into an internal combustion engine make the sensor read lean and and just enter more gasoline? John- John1397
- Thread
-
- Tags
- Hydrogen Introduction Oxygen
- Replies: 4
- Forum: Materials and Chemical Engineering
-

Liquid oxygen expansion in vaccum
Homework Statement There are 2 containers.Outside temperatue is -190 degree celcius.(boiling point of liquid oxygen is -180 degree celcius).One is filled with vacuum and can have a minimum height of 6000m and other is filled with liquid oxygen (1140 kg/m3 density) at -190 degree celcius...- anubodh
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
How to Generate Hydrogen and Oxygen
I have generated hydrogen and oxygen from salt water and water from batteries and have used stainless steel, copper, and aluminum electrodes using electricity, but the electrodes keep disappearing to fast. Is there a way to generate hydrogen and oxygen without this problem or by maybe changing...- John1397
- Thread
- Replies: 5
- Forum: General Engineering
-
K
Chemistry: Methane - Oxygen Combustion Problem - Help needed please.
Hello, Please help me with the following problem. Homework Statement 0.10 Liter of Methane (CH4) gas, at a temperature of 25C and pressure of 744mmHg, react with Oxygen (O2) gas, at constant pressure. The heat released during the process is then transferred to an "ice...- kokoman
- Thread
-
- Tags
- Chemistry Combustion Methane Oxygen
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
W
Compressed oxygen for jet engine (?)
as jet engines lose there productivity at altitude due to thining air/oxygen , why isn't injecting compressed air directly along side the fuel a good idea ? , is this already being done ? would /does this not increase ceiling height and allow sub orbit levels or possible leave atmosphere...- willis hallis
- Thread
-
- Tags
- Compressed Engine Jet Jet engine Oxygen
- Replies: 26
- Forum: General Engineering
-
C
How does singlet oxygen work in pericyclics?
and here's the diagram for the 1O2 dienophile: Its the 1E+g the one I'm interested in. I see that its single electrons are in a couple of degenerate pi orbitals. I'm having trouble figuring out what the LUMO is here. Does it even matter that I have 2 LUMOs? I kinda suspect, that would make it...- CrimpJiggler
- Thread
- Replies: 1
- Forum: Chemistry
-
C
Oxygen forms ions with element X
A piece of unidentified element X reacts with oxygen to form an ionic compound with the chemical formula X2O3. Which of the following elements is the most likely identity of X? A) Ba B) Cs C) In D) P E) Zn I am doing exam practice questions... -
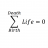
Determine the volume of oxygen
Homework Statement Determine the volume of oxygen gas required to completely combust 3.5mole of propane in a barbecue at standard pressure and 28.0°C. Homework Equations ##PV = nRT## The Attempt at a Solution My first question is, is the work I've done here still correct...- STEMucator
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
O
Why magnet can attract liquid oxygen but not aluminium?both paramagnet
A magnet only attract ferromagnetic material because they have magnetic domains which will align to form magnetic dipole when external magnetic field applied. Paramagnet don't have domains. Due to the presence of some unpaired electron, they will cause the realignment of the electron path when...- Outrageous
- Thread
- Replies: 4
- Forum: Electromagnetism
-
T
Solving Oxygen Flow Rate Issue with One Cylinder
I am trying to solve an oxygen flow rate issue that has me confused. The goal is to have one portable oxygen cylinder (1500psi) provide 2 lines of oxygen at 15 litres/min. The current oxygen cylinder is fitted with a regulator and flow meter that provides 0-15 litres/min. I understand...- tacticsnz
- Thread
- Replies: 4
- Forum: Other Physics Topics
-
F
Atmospheric stoichiochemistry: what mass of oxygen gas is produced?
Homework Statement Oxgyen gas generated in the thermal decomposition of potassium chlorate is collected over water. At 24 C and an atmospheric pressure of 762 mm Hg ((101.6 kPa), the volume of gas collected is 0.128 L. The vapor pressure of water is 22.4 torr (2.98 kPa). What mass of oxygen is...- Fifty
- Thread
-
- Tags
- Atmospheric Gas Mass Oxygen produced
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
S
How much potassium chloride to make 6L of oxygen gas ?
1. Calculates the weight of potassium chloride needed to prepare 6.00L of oxygen gas at the temperature of 24.0 C and 0,950 atm. 2. The equation is : KClO3 = KCl + 3/2O2 3. A hint please ?- sophiia98
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
E
Universal gas constant for 1 kg oxygen
We know, the unit for R=... J / mol K But in a math problem, they asked for universal gas constant for 1 kg of oxygen... They gave pressure,density and temperature... They got the unit of R J/kg K as they used p=(density) R T What does R mean here? But I used pv=nRT...- Ezio3.1415
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
A
Solving Oxygen Contamination in a Glove Box with Clathrates
I'm working with clathrate compounds in a glove box filled with Argon. My problem is that there always remains a small amount of oxygen in the glove box even after multiple purges. This oxygen binds and makes an oxide layer on my clathrates. I don't know how to avoid this and am trying to find...- AWood16
- Thread
- Replies: 4
- Forum: Materials and Chemical Engineering
-
B
The figure shows a PV diagram for 2.69 mole of oxygen gas in a sealed
Homework Statement The figure shows a PV diagram for 2.69 mole of oxygen gas in a sealed container. The temperature of state 1 is 21 C. Pressure is Pi. What is T3 and T4? http://s74.photobucket.com/user/teysure/media/phyiscs.png.html?sort=3&o=0 my attempt at solving the solution... -
W
What state would oxygen be at?
Lets say we have oxygen with a temperature of 280 K at an atmospheric pressure of 50 bar. What would the state of oxygen be in? Gas Liquid Solid- willstaruss22
- Thread
- Replies: 4
- Forum: Chemistry
-
A
Principal Quantum Number of the Excited Oxygen atom.
Normally, Oxygen has 8 electrons in its neutral form that is 1s2 2s2 2p4. In this case, its principal quantum number (n) is two. But what happens if it got excited and its electronic configuration becomes 1s2 2s2 2p3 3s1? In this case, Is the principal quantum number (n) of oxygen two or... -
R
Simple experiment to measure atmospheric Oxygen
I am doing a biology project in which I needed to measure the O2 in the test chamber to determine how much activity I had. The Oxygen sensor I bought on eBay was long expired and useless. O2 instruments were at least $120 used. So I began looking for a simple cheap solution to do it with common... -

How does the sun burn without oxygen?
On Earth, fire needs oxygen. If space has no oxygen, how does the sun continue to burn? Thanks! :) Ω- Anna Blanksch
- Thread
- Replies: 5
- Forum: Astronomy and Astrophysics
-

The required energy to split up an Oxygen atom
Homework Statement "Calculate how much energy that is required for a oxygen atom 168O to split it up into 4 α-particles" The Attempt at a Solution 1: (8 * 1,00727646688)u + (8* 1,00866491578)u = 16,12753105 u The first one is the Oxygen's 8 protons and the second one is its 8 neutrons...