- #36
Devin-M
- 994
- 761
I made this to illustrate.
After 2, same weight, compressed samples of C02 at constant pressure transfer the same amount of energy to sub-zero C environments in separate experiments (one at 5MPa and the other at 2.5MPa, both starting at 125C), at the end of the experiments, the 5MPa CO2 sample is 25C warmer than the 2.5MPa CO2 sample... the 2.5MPa sample follows the log mean formula so wouldn't the 5MPa sample be warmer than the log mean formula implies, on average?

After 2, same weight, compressed samples of C02 at constant pressure transfer the same amount of energy to sub-zero C environments in separate experiments (one at 5MPa and the other at 2.5MPa, both starting at 125C), at the end of the experiments, the 5MPa CO2 sample is 25C warmer than the 2.5MPa CO2 sample... the 2.5MPa sample follows the log mean formula so wouldn't the 5MPa sample be warmer than the log mean formula implies, on average?
Chestermiller said: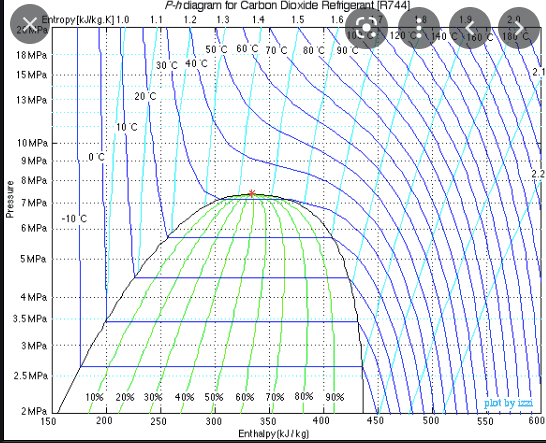
Last edited: