- #1
lee123456789
- 93
- 5
- Homework Statement
- dont know what this means
- Relevant Equations
- in the post
why it is first law of thermodynamics useful in developing the non-flow energy equation and steady-flow energy equation.
First Law Equation (FLE) =
1
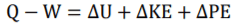
1.1
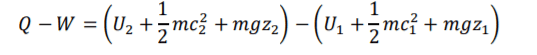
Non-Flow Equation =
2
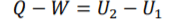
Steady-Flow Equation =
3
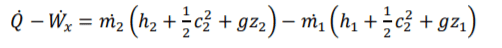
Specific Entrapy of Steady-Flow
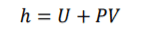
4
Mass Flow
5
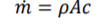
I understand (i think) but can't explain.
Im understand it (i think) but finding hard to explain can somebody help. is what i put fitting the question?, how do i make it better? can somebody help it
FLE can be expressed as 1.1.
The first law of thermodynamics (1) contains (kinetic energy change) & PE (potential energy change). Theses contain contain two equation components that if u minus second half from first half will equal U,KE or PE. This allows (1) RHS to be broken down and reranged into two individual equation components forming (1.1).
Non-Flow
The system is closed so is not effected by external factors so addaction components need not be included into the eqaution. FLE (1) under non-flow conditions Δ𝐾 and Δ𝑃E are negligible, so equal ( 0). Under theses conditon Δ𝐾 and Δ𝑃E are omitted from the equation forming equation (2)
Steady-Flow
for steady flow systems, the system is open so if effected by air flow so the equation is required to change to accommodate this factor. Mass flow change of area, speed or density cause mass flow change change. There is 2 mass flows, mass flow in and out. FLE (1.1) m for both equation components will be label m1 or m2. m effect all conditions within both equation components so can be brought out sided the brackets.
(U) specific internal energy is effected by specific entraphy and pressure & specific pressure. rearrange the equation allows you to determine specific entraphy of the system in and out. Specific entraphy is important for steady-flow and allows you to devople a key component of the eqaution.
I don't know what Wx (work) is and how to explain it?
First Law Equation (FLE) =
1
1.1
Non-Flow Equation =
2
Steady-Flow Equation =
3
Specific Entrapy of Steady-Flow
4
Mass Flow
5
I understand (i think) but can't explain.
Im understand it (i think) but finding hard to explain can somebody help. is what i put fitting the question?, how do i make it better? can somebody help it
FLE can be expressed as 1.1.
The first law of thermodynamics (1) contains (kinetic energy change) & PE (potential energy change). Theses contain contain two equation components that if u minus second half from first half will equal U,KE or PE. This allows (1) RHS to be broken down and reranged into two individual equation components forming (1.1).
Non-Flow
The system is closed so is not effected by external factors so addaction components need not be included into the eqaution. FLE (1) under non-flow conditions Δ𝐾 and Δ𝑃E are negligible, so equal ( 0). Under theses conditon Δ𝐾 and Δ𝑃E are omitted from the equation forming equation (2)
Steady-Flow
for steady flow systems, the system is open so if effected by air flow so the equation is required to change to accommodate this factor. Mass flow change of area, speed or density cause mass flow change change. There is 2 mass flows, mass flow in and out. FLE (1.1) m for both equation components will be label m1 or m2. m effect all conditions within both equation components so can be brought out sided the brackets.
(U) specific internal energy is effected by specific entraphy and pressure & specific pressure. rearrange the equation allows you to determine specific entraphy of the system in and out. Specific entraphy is important for steady-flow and allows you to devople a key component of the eqaution.
I don't know what Wx (work) is and how to explain it?