- #1
- 3,012
- 42
Let's say you have 2 batteries, both 1.5 V. If you put your red voltmeter lead on the + of one battery and your black voltmeter lead on the - of the other battery, and assuming the two batteries are not touching, the voltage you read is zero. I know this is a simple question, but why? Why don't you read some voltage, say 1.5 V?
I've always thought of voltage as a potential field that is more negative on one end and more positive on another. I'd envision the battery having excess electrons on the negative end of the battery and they would be attracted to any more positive electrical potential, kinda like this picture:
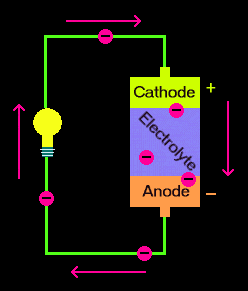
http://www.qrg.northwestern.edu/projects/vss/docs/power/2-how-do-batteries-work.html
It seems like the electrons on the negative end of the battery should be able to flow to the positive end of another battery when given a conductive path, even when the batteries aren't connected - kinda like lightning. But clearly that isn't the case. So why don't the electrons go from one battery to another unless they're connected?
I've always thought of voltage as a potential field that is more negative on one end and more positive on another. I'd envision the battery having excess electrons on the negative end of the battery and they would be attracted to any more positive electrical potential, kinda like this picture:
http://www.qrg.northwestern.edu/projects/vss/docs/power/2-how-do-batteries-work.html
It seems like the electrons on the negative end of the battery should be able to flow to the positive end of another battery when given a conductive path, even when the batteries aren't connected - kinda like lightning. But clearly that isn't the case. So why don't the electrons go from one battery to another unless they're connected?
Last edited by a moderator: