- #1
obad
- 12
- 0
Hi guys,
I have a question to you that has been bothering me for a while now.
It is about adiabatic flow in a duct or more specifically in the air intake of a jet engine.
In lectures and textbooks it is always stated that the total temperature in an adiabatic
flow does not change. Hence the total temperature at the inlet and outlet of a jet engine
air intake is the same. However, the total pressure reduces due to friction. This kind of
flow is also known as Fanno flow.
So the common explanation is, that friction does not alter the total temperature. But
when having a look at the energy equation for viscous flows I cannot agree with this
statement.
I wrote down the energy equation in integral form for the total internal energy e.
If we say the flow is stationary, equal pressure at inlet and outlet and adiabatic, then
the instationary term on the left side and the temperature source term and temperature
conduction term as well as the pressure term on the right side can be
cancelled out.

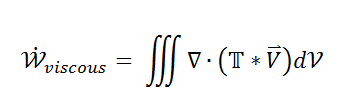 So what remains is the left side telling me the net amount of energy that leaves my volume
So what remains is the left side telling me the net amount of energy that leaves my volume
and on the right side the energy term due to shear stress.
Heating of the flow due to shear stress can usually be neglected f or low Mach number flows
where the velocity gradients close to the wall are small.
But if I take it accurately then the viscous heating should change the energy flow through
my control volume, right? At least for supersonic air intakes this should make a difference,
however in textbooks the total temperature stays constant.
I appreciate your help!
I have a question to you that has been bothering me for a while now.
It is about adiabatic flow in a duct or more specifically in the air intake of a jet engine.
In lectures and textbooks it is always stated that the total temperature in an adiabatic
flow does not change. Hence the total temperature at the inlet and outlet of a jet engine
air intake is the same. However, the total pressure reduces due to friction. This kind of
flow is also known as Fanno flow.
So the common explanation is, that friction does not alter the total temperature. But
when having a look at the energy equation for viscous flows I cannot agree with this
statement.
I wrote down the energy equation in integral form for the total internal energy e.
If we say the flow is stationary, equal pressure at inlet and outlet and adiabatic, then
the instationary term on the left side and the temperature source term and temperature
conduction term as well as the pressure term on the right side can be
cancelled out.
and on the right side the energy term due to shear stress.
Heating of the flow due to shear stress can usually be neglected f or low Mach number flows
where the velocity gradients close to the wall are small.
But if I take it accurately then the viscous heating should change the energy flow through
my control volume, right? At least for supersonic air intakes this should make a difference,
however in textbooks the total temperature stays constant.
I appreciate your help!