- #1
pkc111
- 225
- 26
- TL;DR Summary
- I am confused about information regarding the effect of light frequency on photocurrent in the Lenard's apparatus.
Pearson Physics 12 states:
"When the light sources have the same intensity but different frequencies, they produce the same maximum current"
However, Phet Simulation Photoelectric Effect seems to show that photocurrent changes with light frequency (eg see below for different photocurrents at 179 nm and 414 nm incident light wavelengths on sodium:

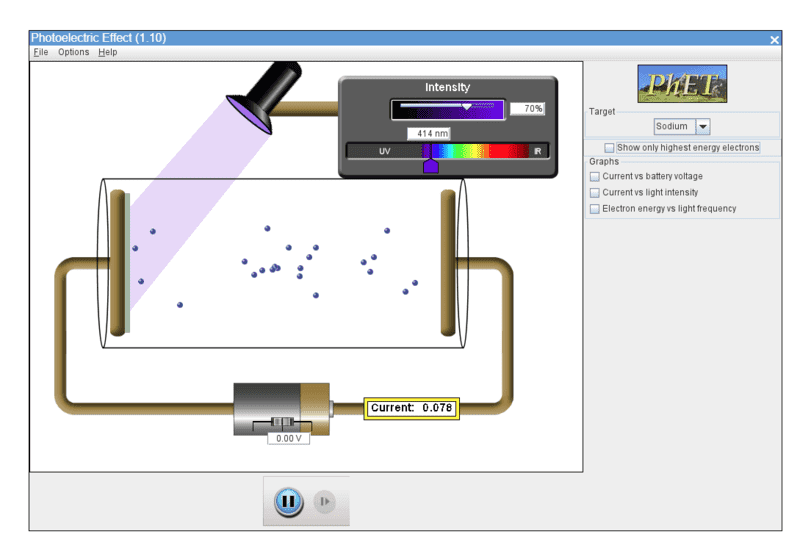
"When the light sources have the same intensity but different frequencies, they produce the same maximum current"
However, Phet Simulation Photoelectric Effect seems to show that photocurrent changes with light frequency (eg see below for different photocurrents at 179 nm and 414 nm incident light wavelengths on sodium: