- #1
jarekduda
- 82
- 5
Stark effect (shifting and splitting spectral lines due to external electric field) is calculated in nearly all QM textbooks as application of perturbation theory (alongside Zeeman).
Wikipedia article ( https://en.wikipedia.org/wiki/Stark_effect ) has a nice figure with n-th level splitting into n-1 uniformly distributed sublevels:
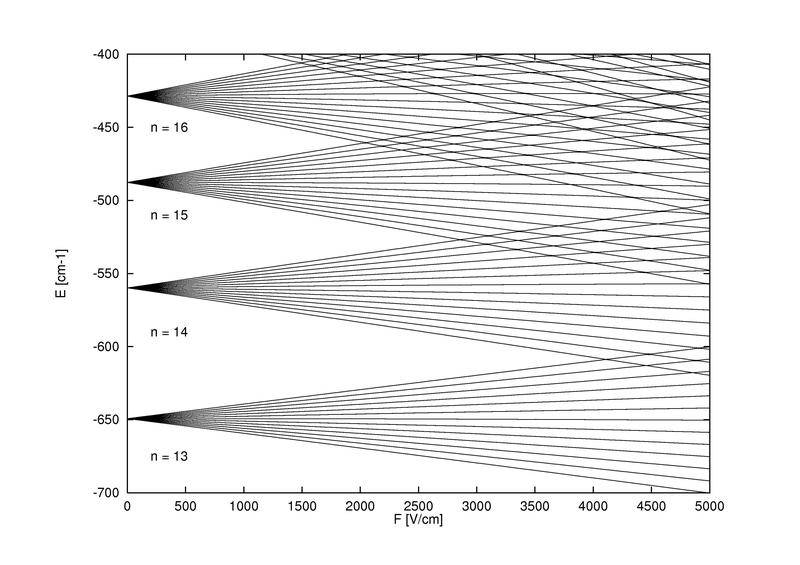
It is hard to find published experimental results - please cite if you know some.
A clear one for Lyman series (2->1, 3->1, 4->1) can be found in historical "Der Starkeffect der Lymanserie" by Rudolf Frerichs, published January 1934 in Annalen der Physic (its editors back then: W. Gerlach, F. Pashen, M. Planck, R. Pohl, A. Sommerfeld and M. Wien), here are its results:
https://dl.dropboxusercontent.com/u/12405967/stark.png
These are clearly not uniformly distributed - how to understand/repair this discrepancy?
Wikipedia article ( https://en.wikipedia.org/wiki/Stark_effect ) has a nice figure with n-th level splitting into n-1 uniformly distributed sublevels:
It is hard to find published experimental results - please cite if you know some.
A clear one for Lyman series (2->1, 3->1, 4->1) can be found in historical "Der Starkeffect der Lymanserie" by Rudolf Frerichs, published January 1934 in Annalen der Physic (its editors back then: W. Gerlach, F. Pashen, M. Planck, R. Pohl, A. Sommerfeld and M. Wien), here are its results:
https://dl.dropboxusercontent.com/u/12405967/stark.png
These are clearly not uniformly distributed - how to understand/repair this discrepancy?
Last edited by a moderator: