- #1
mech-eng
- 828
- 13
"The saturation line for a given substance is the combination of temperature and pressure where 100% vapor exists. At any point below this line, there will be a mixture of vapor and liquid, until a point is reached when only the liquid phase exists."
I don't understand above passage from @SteamKing's post. Is saturation line= saturated liquid line + saturated vapor line, as in the picture?
If you have a mixture, can you use a saturated liquid table?
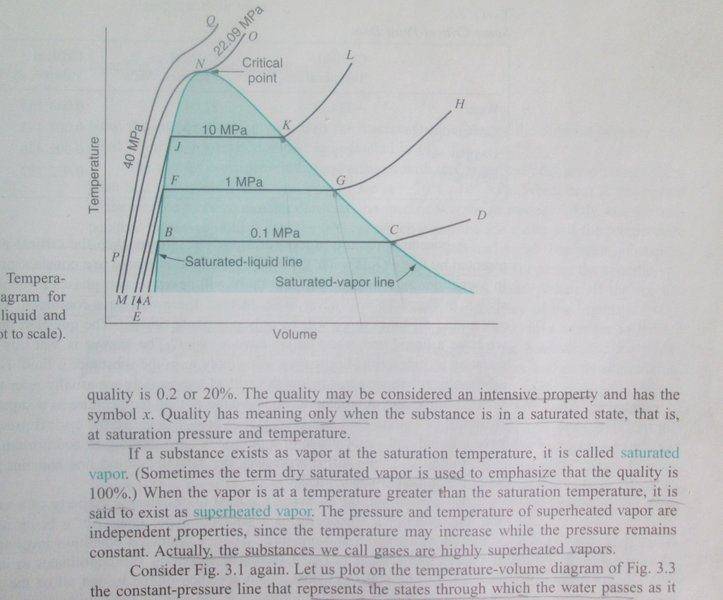
Source: Introduction to Engineering Thermodynamics by Sonntag/Borgnakke.
Thank you.
I don't understand above passage from @SteamKing's post. Is saturation line= saturated liquid line + saturated vapor line, as in the picture?
If you have a mixture, can you use a saturated liquid table?
Source: Introduction to Engineering Thermodynamics by Sonntag/Borgnakke.
Thank you.