- #1
mesa
Gold Member
- 695
- 38
An electron as shown by the Stern Gerlach experiment behaves like a dipole (albeit only in one of two states). I have been trying to figure out how this is so and drew up the following sketches. A few assumptions were made about electrons such as 'distribution' of charge assuming static equilibrium resulting in a shell of charge. Charge distribution could also be spread out evenly or by some gradient from the center however the general result would still be the same.
Figure one shows the F.o.R. with our shell rotating about the x-axis in a clockwise direction when viewed from the positive x towards the negative. This rotation is the same for all three figures. Figure 2 is a cutaway about the y/z plane and a slice to it's right with direction of rotation shown again. So essentially the rotation of our charge distribution of some radius will act like a dipole just like a current loop with this case having a north pole facing the positive x direction and the south the negative x for all 'slices'.
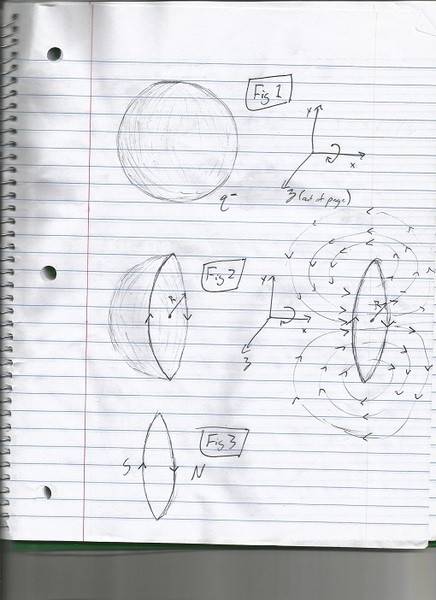
The issue here is that an electrons dipole could point in any direction depending on its rotation and the magnitude of the Bf of each dipole would similarly be dependent on the speed of that rotation for whatever F.o.R. is used. As I understand it the Stern Gerlach experiments show otherwise as the electron can have only on of two possible directions of the dipole and of equal magnitude. Is this correct?
Figure one shows the F.o.R. with our shell rotating about the x-axis in a clockwise direction when viewed from the positive x towards the negative. This rotation is the same for all three figures. Figure 2 is a cutaway about the y/z plane and a slice to it's right with direction of rotation shown again. So essentially the rotation of our charge distribution of some radius will act like a dipole just like a current loop with this case having a north pole facing the positive x direction and the south the negative x for all 'slices'.
The issue here is that an electrons dipole could point in any direction depending on its rotation and the magnitude of the Bf of each dipole would similarly be dependent on the speed of that rotation for whatever F.o.R. is used. As I understand it the Stern Gerlach experiments show otherwise as the electron can have only on of two possible directions of the dipole and of equal magnitude. Is this correct?