- #1
TheCelt
- 24
- 5
Hello
I am trying to understand how to write the reduced mass into atomic mass units but i am confused how it was done.
The equation is
$$m_1m_2/(m_1+m_2)$$
For two similar masses in my particular case i have:
$$m^2 / 2m = 1/2 * m$$
Then to convert to atomic mass units, the book says it gives:
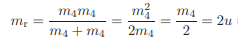
But how did they get that value? I had $$1/2 * u$$My conversion must be failing me, i am not sure how they got that value..
I am trying to understand how to write the reduced mass into atomic mass units but i am confused how it was done.
The equation is
$$m_1m_2/(m_1+m_2)$$
For two similar masses in my particular case i have:
$$m^2 / 2m = 1/2 * m$$
Then to convert to atomic mass units, the book says it gives:
But how did they get that value? I had $$1/2 * u$$My conversion must be failing me, i am not sure how they got that value..