- #1
plazprestige
- 33
- 0
In my physical chemistry course, we are learning about the Schrödinger Equation and were introduced to the Hamiltonian Operator recently. We started out with the simple scenario of a particle in 1D space. Our professor's slide showed the following "derivation" to arrive at the expression for the kinetic energy component of the Hamiltonian (see attachment).
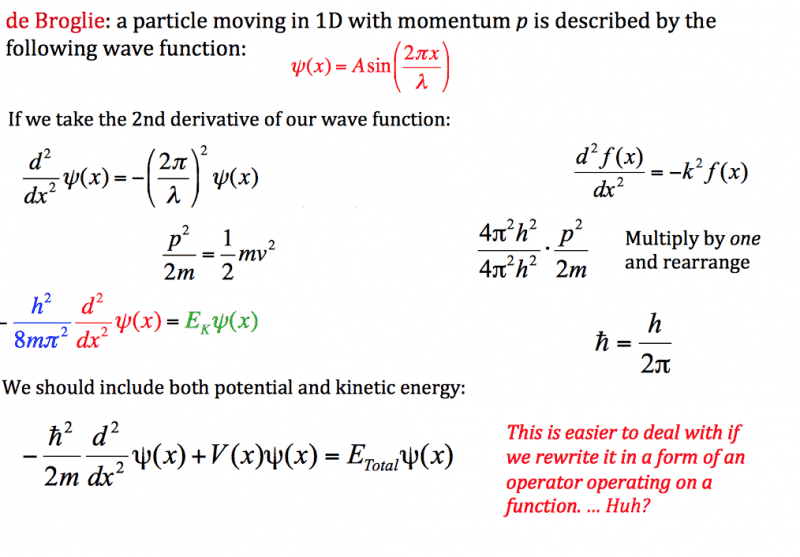
I do not understand how the professor arrived at the eigenfunction-eigenvalue equation for the kinetic energy involving Planck's constant. I understand that kinetic energy is equal to momentum squared divided by twice the mass, of course, but nothing beyond that in the slide. If someone could explain this, I would appreciate it. Please note that I am not a physics major, so any mathematics more advanced than calculus may be beyond me (I do understand operators and eigenfunctions, however).
I do not understand how the professor arrived at the eigenfunction-eigenvalue equation for the kinetic energy involving Planck's constant. I understand that kinetic energy is equal to momentum squared divided by twice the mass, of course, but nothing beyond that in the slide. If someone could explain this, I would appreciate it. Please note that I am not a physics major, so any mathematics more advanced than calculus may be beyond me (I do understand operators and eigenfunctions, however).