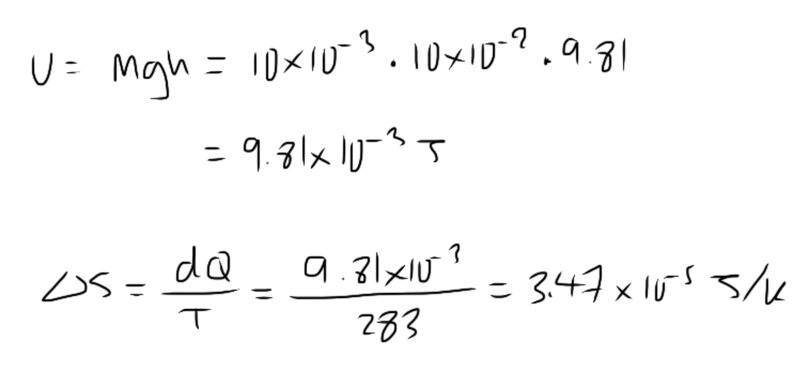- #1
so_gr_lo
- 69
- 10
- Homework Statement
- What is the probability that a 10g pencil lying on a table will spring spontaneously 10cm into the air at the expense of thermal energy of its surroundings which are at 15°C. What do you conclude from your result?
I’m guessing I’m supposed to calculate the gravitational potential energy, which comes from the thermal energy of the surroundings, but I’m not sure how that relates to probability. In my course I’ve only been given entropy equations, not sure how it relates to probability
- Relevant Equations
- dS = dQ/T
U = mgh
I