- #1
misko
- 46
- 0
In rovibrational transitions we have following selection rules
$$ \Delta v = \pm 1 $$
$$ \Delta J = \pm 1 $$
where ##v## is the vibrational quantum number and ##J## is rotational quantum number.
Now based on whether ##\Delta J## changes to +1 or -1 we have two branches of spectroscopic lines.
They are defined as following:
- if transition happens such that ##\Delta J = +1## that goes in R-branch
- if transition happens such that ##\Delta J = -1## that goes in P-branch
It is also said (eg. https://en.wikipedia.org/wiki/Rotational–vibrational_spectroscopy) that R branch is on the higher frequency side of the Q branch and P branch is on the lower frequency side. This means that the lines in R branch have more photon energy (eg. less wavelength) than lines in the P branch.
So far so good.
But notice how I emphasized transition word above? That's because in every site/book I looked at I see they use word transition without specifying whether it's absorption or emission. It seems as if it doesn't matter but looks like it does.
If we have absorption and ##\Delta J = +1## (say J goes from 0 to 1) then that is R branch. But if we have emission (the same transition in opposite direction where J goes from 1 to 0) we will have ##\Delta J = -1##. Now both of these transitions must be of the same energy but which should go to the R branch? I conclude it must be that the transition specified above in the definition is absorption and not emission.
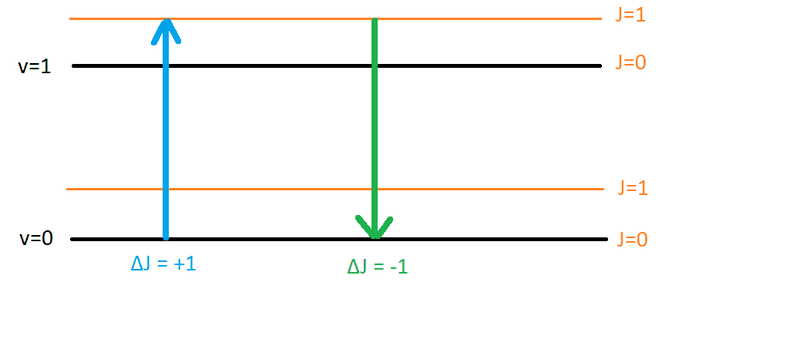
So why I couldn't find in any resource this? Why did I have to lose 3-4 hours on this trying to figure out all this rather than having a precise definition in the first place? When I see transition I usually first think of emission so I started working examples on my own to understand this R and P branches concepts but I couldn't match it with the definition.
I am disappointed by the physics literature and a loose way of defining things. Or could it be me? Am I missing something from my discussion here? It could be because of lack of sleep I am having these days but I am not seeing it.
$$ \Delta v = \pm 1 $$
$$ \Delta J = \pm 1 $$
where ##v## is the vibrational quantum number and ##J## is rotational quantum number.
Now based on whether ##\Delta J## changes to +1 or -1 we have two branches of spectroscopic lines.
They are defined as following:
- if transition happens such that ##\Delta J = +1## that goes in R-branch
- if transition happens such that ##\Delta J = -1## that goes in P-branch
It is also said (eg. https://en.wikipedia.org/wiki/Rotational–vibrational_spectroscopy) that R branch is on the higher frequency side of the Q branch and P branch is on the lower frequency side. This means that the lines in R branch have more photon energy (eg. less wavelength) than lines in the P branch.
So far so good.
But notice how I emphasized transition word above? That's because in every site/book I looked at I see they use word transition without specifying whether it's absorption or emission. It seems as if it doesn't matter but looks like it does.
If we have absorption and ##\Delta J = +1## (say J goes from 0 to 1) then that is R branch. But if we have emission (the same transition in opposite direction where J goes from 1 to 0) we will have ##\Delta J = -1##. Now both of these transitions must be of the same energy but which should go to the R branch? I conclude it must be that the transition specified above in the definition is absorption and not emission.
So why I couldn't find in any resource this? Why did I have to lose 3-4 hours on this trying to figure out all this rather than having a precise definition in the first place? When I see transition I usually first think of emission so I started working examples on my own to understand this R and P branches concepts but I couldn't match it with the definition.
I am disappointed by the physics literature and a loose way of defining things. Or could it be me? Am I missing something from my discussion here? It could be because of lack of sleep I am having these days but I am not seeing it.