- #1
Radhakrishnam
- 63
- 2
The expression relating the mean number of molecules with velocities in the range v and v + dv and position r and r + dr is given by
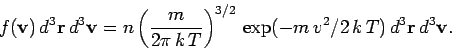
where n = N/V is the number density of molecules.
My question is: Since LHS is an integer, how do we ascertain the RHS is an integer, since it involves pi and an exponential factor that are irrational numbers? How do we ascertain the product of the irrational numbers always leads to an integer?
where n = N/V is the number density of molecules.
My question is: Since LHS is an integer, how do we ascertain the RHS is an integer, since it involves pi and an exponential factor that are irrational numbers? How do we ascertain the product of the irrational numbers always leads to an integer?