- #1
rwooduk
- 762
- 59
This is what we have been given in class:
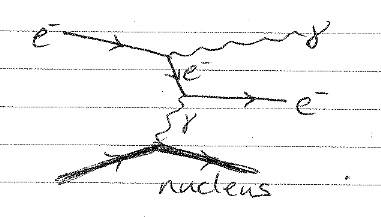
I'm a little confused as the electron seems to emit two gamma rays? I understand that the process can't occur if there isn't interaction with a nucleus due to conservation of momentum. Please could someone confirm that the top gamma is the initial release due to deceleration / acceleration, and the lower gamma is there to satisfy conservation laws.
Thanks for any help.
I'm a little confused as the electron seems to emit two gamma rays? I understand that the process can't occur if there isn't interaction with a nucleus due to conservation of momentum. Please could someone confirm that the top gamma is the initial release due to deceleration / acceleration, and the lower gamma is there to satisfy conservation laws.
Thanks for any help.