- #1
oem7110
- 151
- 0
If I place a super magnet under the bottom of my water bottle, will it magnetize the water I drink everyday?
Does anyone have any suggestions?
Thanks in advance for any suggestions
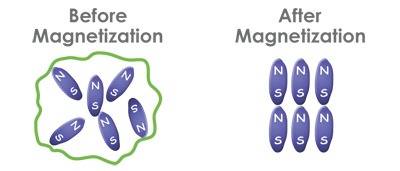
"After magnetization, the molecules line up in sequence “+-+-“ resulting in reduced surface tension, reduced viscosity, increased dissolvability, increased permeability and increased oxygen content hence making nutrients more readily available to our body, plants and soils. Water is then said to be biologically “alive”."
Does anyone have any suggestions?
Thanks in advance for any suggestions
"After magnetization, the molecules line up in sequence “+-+-“ resulting in reduced surface tension, reduced viscosity, increased dissolvability, increased permeability and increased oxygen content hence making nutrients more readily available to our body, plants and soils. Water is then said to be biologically “alive”."