- #1
ChiralSuperfields
- 1,225
- 132
- Homework Statement
- Please see below
- Relevant Equations
- Conservation of energy
For this,

The solution is,
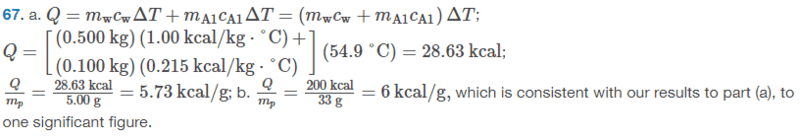
However, I am not sure why they did part(a) like that. I thought we would do it like this:
##Q_{nut} + Q_{w} = 0## since calorimeter is ideal so energy is conserved in the nut-water system
##Q_{nut} =-Q_{w}##
##Q_{nut} = -(0.500)(4184)(54.9) = -1.15 \times 10^5 J##
Therefore, the heat lost by the nut is ##Q_{nut} = 1.15 \times 10^5 J = 27.45 \frac{kcal}{5.00g} = 5.49 \frac{kcal}{g}##
However, I don't understand their method for part(a), is there a mistake in mine?Many thanks!
The solution is,
However, I am not sure why they did part(a) like that. I thought we would do it like this:
##Q_{nut} + Q_{w} = 0## since calorimeter is ideal so energy is conserved in the nut-water system
##Q_{nut} =-Q_{w}##
##Q_{nut} = -(0.500)(4184)(54.9) = -1.15 \times 10^5 J##
Therefore, the heat lost by the nut is ##Q_{nut} = 1.15 \times 10^5 J = 27.45 \frac{kcal}{5.00g} = 5.49 \frac{kcal}{g}##
However, I don't understand their method for part(a), is there a mistake in mine?Many thanks!