- #1
eddywalrus
- 25
- 0
Here is a screenshot from a page from a textbook that explains how to derive the ideal gas law:
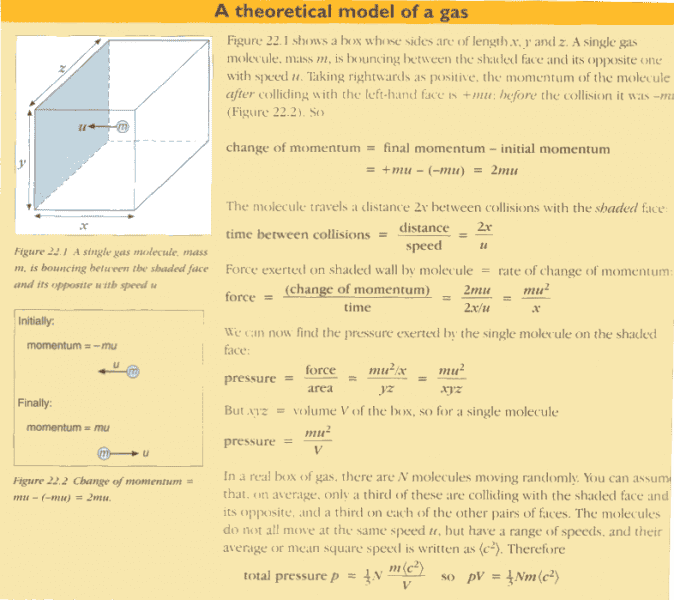
In the third bold line, I don't understand how "time" in force = (change of momentum)/(time) is equal to 2x/u (the time it takes for the particle to travel to the opposite face and back again) -- I always assumed that:
impulse = Force x time
change in momentum = Force x time
where time in this case refers to the time of contact between the two colliding objects? Furthermore, since the particle doesn't change its momentum over the duration of traveling to the opposite face and back again (but instead changes momentum during its collision with the container wall), shouldn't the "time" in this case refer to the time of collision?
Thank you so much for all your help!
In the third bold line, I don't understand how "time" in force = (change of momentum)/(time) is equal to 2x/u (the time it takes for the particle to travel to the opposite face and back again) -- I always assumed that:
impulse = Force x time
change in momentum = Force x time
where time in this case refers to the time of contact between the two colliding objects? Furthermore, since the particle doesn't change its momentum over the duration of traveling to the opposite face and back again (but instead changes momentum during its collision with the container wall), shouldn't the "time" in this case refer to the time of collision?
Thank you so much for all your help!