- #1
Klacid
- 2
- 0
Hey guys i wanted to know how make some chemicals when i have an equation, for example:
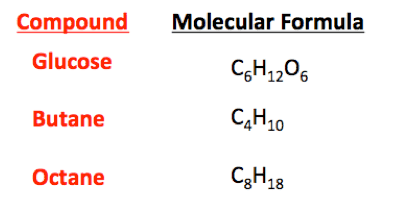
First of all, you don't have any equations in the graphic above. These are called the molecular formulas for the various compounds shown.Klacid said:Hey guys i wanted to know how make some chemicals when i have an equation, for example:

To write a chemical equation, you will need to know the reactants (substances that are combined) and the products (substances that are produced). Write the reactants on the left side of the equation and the products on the right side. Use plus signs (+) to separate multiple reactants and products, and an arrow (→) to show the direction of the reaction. For example: A + B → C + D.
Coefficients are numbers that are placed in front of a chemical formula to indicate the number of molecules or moles of that substance. Subscripts are numbers that are written below and to the right of a chemical symbol to indicate the number of atoms of that element in a molecule. Coefficients affect the entire molecule, while subscripts only affect the atom they are next to.
To balance a chemical equation, you need to make sure that the number of atoms of each element is the same on both sides of the equation. Start by balancing elements that appear only once on each side of the equation and then work your way through the other elements until the equation is balanced. You can add coefficients in front of the formulas or change the subscripts (only if the formula represents a polyatomic ion).
A chemical reaction is a process in which one or more substances (reactants) are transformed into new substances (products) with different chemical and physical properties. This transformation is caused by the breaking and forming of chemical bonds between atoms. Chemical reactions can be described by writing a chemical equation.
A balanced chemical equation is important because it accurately shows the quantities and types of substances involved in a chemical reaction. It also follows the law of conservation of mass, which states that matter cannot be created or destroyed, only rearranged. A balanced equation ensures that the same number of atoms of each element are present on both sides of the equation, which is necessary for a reaction to occur.