- #1
swankdave
- 2
- 0
Try as I may, I can't figure out how to even phrase the following in a way to google it, so I apologize in advance if it is a trivial question, I am no physicist.
What I am trying to discern is if there are very very small shifts in the perceived charge of an atom over very very small amounts of time.
Consider an electron (beta radiation) passing a helium atom. (for the sake of argument, passing at a distance of the width of the helium atom from the atom's nucleus, and moving at 1/2 the speed of the electrons of the helium atom, with respect to the helium atom nucleus) Let us consider the force exerted between the electron and the atom, one heliums width before and after the electrons closest point of approach to the helium atom.
(figure 1)
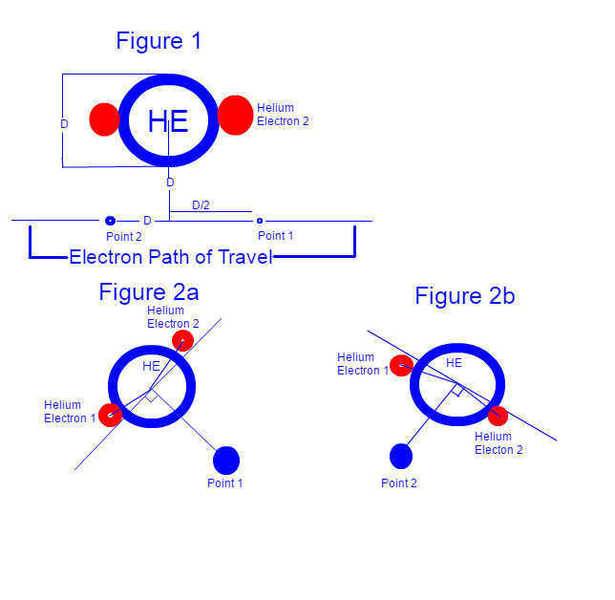
With Classic modeling, as I understand it, we image that the electrons of the helium atom are, at all times, equidistant to the nucleus and diametrically opposed. As such, the electromagnetic field experienced at both our points of consideration on the electron's path are identical in magnitude (and rather close to zero), but not direction, as one would expect.
As I understand it however, there are reasons why electrons would NOT be perfectly diametrically opposed and equidistant from the nucleus (temperature, vibrational axis movement, etc.) and so, given that the electrons of the helium atom would have changed positions as our radiation electron passed the atom, the magnitude of the force exerted between the helium atom and the electron would not necessarily be equal. The proposal is that the movements of electrons within an atom is slightly messy, leading to tiny variations of directionally perceived charge of the atom.
To look at it another way, say the electrons in the helium atom were able to move 5deg off of diametrically opposed. If both electrons were 92.5 deg away from our passing electron (when measured from the nucleus) at our first point of consideration, and 87.5 deg away from our passing electron at our second point of consideration, then our electron would experience a pulling force at the first point and a pushing force at the second point.
(figure 2a and 2b)

I am not suggesting any violation of conservation of energy here, merely that there would be tiny energy exchanges between atoms due to this type of momentary imbalance, with a net change of zero over time.
To my understanding, if the "messy" model is correct, it is likely an amount of force too small to measure with current instrumentation, but, if possible, I would love a way to prove if such "momentary imbalances" exist.
Thanks!
What I am trying to discern is if there are very very small shifts in the perceived charge of an atom over very very small amounts of time.
Consider an electron (beta radiation) passing a helium atom. (for the sake of argument, passing at a distance of the width of the helium atom from the atom's nucleus, and moving at 1/2 the speed of the electrons of the helium atom, with respect to the helium atom nucleus) Let us consider the force exerted between the electron and the atom, one heliums width before and after the electrons closest point of approach to the helium atom.
(figure 1)
With Classic modeling, as I understand it, we image that the electrons of the helium atom are, at all times, equidistant to the nucleus and diametrically opposed. As such, the electromagnetic field experienced at both our points of consideration on the electron's path are identical in magnitude (and rather close to zero), but not direction, as one would expect.
As I understand it however, there are reasons why electrons would NOT be perfectly diametrically opposed and equidistant from the nucleus (temperature, vibrational axis movement, etc.) and so, given that the electrons of the helium atom would have changed positions as our radiation electron passed the atom, the magnitude of the force exerted between the helium atom and the electron would not necessarily be equal. The proposal is that the movements of electrons within an atom is slightly messy, leading to tiny variations of directionally perceived charge of the atom.
To look at it another way, say the electrons in the helium atom were able to move 5deg off of diametrically opposed. If both electrons were 92.5 deg away from our passing electron (when measured from the nucleus) at our first point of consideration, and 87.5 deg away from our passing electron at our second point of consideration, then our electron would experience a pulling force at the first point and a pushing force at the second point.
(figure 2a and 2b)
I am not suggesting any violation of conservation of energy here, merely that there would be tiny energy exchanges between atoms due to this type of momentary imbalance, with a net change of zero over time.
To my understanding, if the "messy" model is correct, it is likely an amount of force too small to measure with current instrumentation, but, if possible, I would love a way to prove if such "momentary imbalances" exist.
Thanks!