- #1
Salmone
- 101
- 13
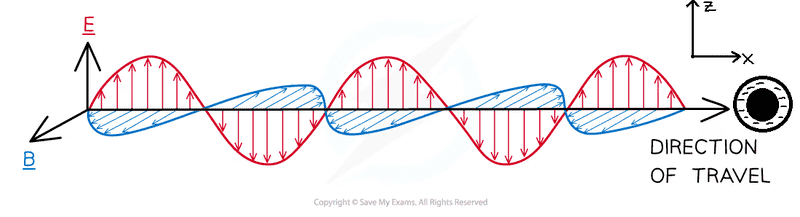
If we have an electromagnetic wave like the one in the picture and a molecule which is, in the image, the small black ball with electron cloud being the part with "minus sign" in it, does the molecule with its cloud start to oscillate, once the EM wave hits it, as an induced electric dipole, along the z-axis or along the x-axis? (The two axes are as drawn in the figure).