- #1
greypilgrim
- 522
- 36
Hi.
The blue curve shows the idealized behaviour of the detected current vs. the acceleration voltage in a Franck-Hertz experiment:
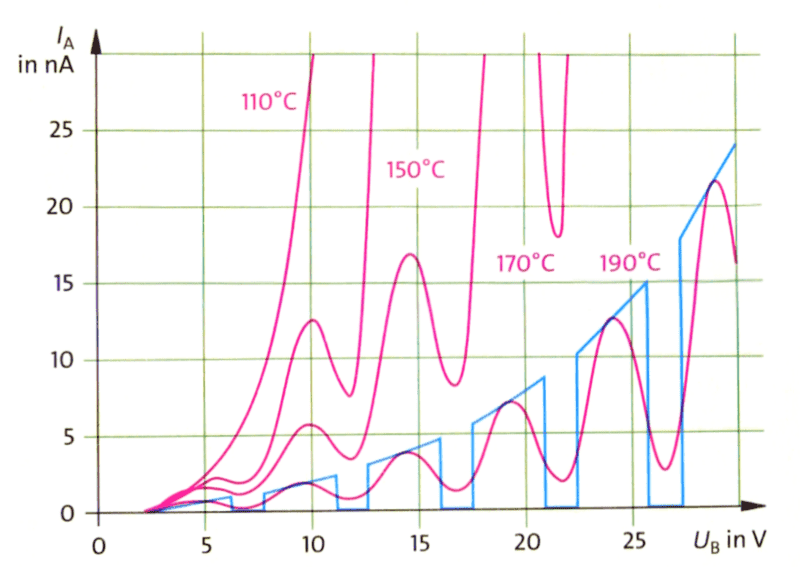
The blue curve shows the idealized behaviour of the detected current vs. the acceleration voltage in a Franck-Hertz experiment:
- It appears that the underlying behaviour is quadratic, why? I calculated the time for an electron to reach the grid at distance ##d## from the hot cathode to be $$t=d\cdot\sqrt\frac{2m_e}{U_B\cdot e}\enspace.$$ Now I'm not sure how to use this. How many electrons are released at the hot cathode per second? Does this depend on ##U_B## as well? I think so, otherwise there shouldn't be an overall increase of the blue curve at all.
- Why isn't the first drop at exactly 4.9 V? Is there still energy needed to release electrons even from a hot cathode? This would probably agree with 1., but is there a simple quantitative relation?
