- #1
warliooo
- 2
- 0
- Homework Statement
- Question: Find the amount of ethanol in gas phase in a tank blanketed by N2 at atmospheric pressure.
An empty tank of 2000 litres at atmospheric pressure and 20 degC is being used to store ethanol waste. The tank is blanketed with nitrogen, and is kept at atmospheric pressure by venting (to atmosphere).
500 litres of ethanol at 70 degrees is added to the tank. By adding 500 litres of ethanol, 500 litres of nitrogen leaves through the vent (atmospheric pressure remained).
Assuming that the ethanol in the tank reaches equilibrium in the new tank and stays at 70 degC, 1) how much ethanol will be in the gas phase within the tank? 2) if another 500 litres of ethanol is added, how much ethanol is vented to the atmosphere?
- Relevant Equations
- Raoults Law
I first found the partial pressure of ethanol at 70 degrees using Antoine coefficients:
Coefficients:
Equation:
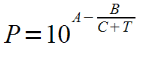
And found a partial pressure of 0.7 bar.
This is the pressure exerted by the vapour on the liquid.
I then found the moles of ethanol at this pressure using the volume of the vessel (2000l-500l):
PV=nRT
n=PV/RT= (0.7*101325)*1500/(8.3143*(273+70))
n=307 moles
Molecular weight of ethanol = 46g/mol
1)
Mass (g) of ethanol in the vapour phase= (307*46)=14130 g
2)
Concentration of ethanol in gas phase:14130/1500=9.42 g/liter vapour
if 500 litres released: 9.42*500/1000 = 4.7 kg released.
Coefficients:
| A | 7.68117 |
| B | 1332.04 |
| C | 199.2 |
Equation:
And found a partial pressure of 0.7 bar.
This is the pressure exerted by the vapour on the liquid.
I then found the moles of ethanol at this pressure using the volume of the vessel (2000l-500l):
PV=nRT
n=PV/RT= (0.7*101325)*1500/(8.3143*(273+70))
n=307 moles
Molecular weight of ethanol = 46g/mol
1)
Mass (g) of ethanol in the vapour phase= (307*46)=14130 g
2)
Concentration of ethanol in gas phase:14130/1500=9.42 g/liter vapour
if 500 litres released: 9.42*500/1000 = 4.7 kg released.