- #1
Robotesco
- 13
- 0
PO43-
I Understand the 4 represents how many oxygen atoms are bonded to the Phosphate. What exactly does the 3 and its - sign represent? By the image, I can tell the difference between the 3 oxygen atoms and the single isolated one, with the double || symbol, has a different electronic composition. Explain why the single | symbol is used and why the isolated O atom uses two ||s. Please also describe the difference between the two electronic compositions. Why does it show 3 pairs of dots on the O33-s and a double pair on the single O2-
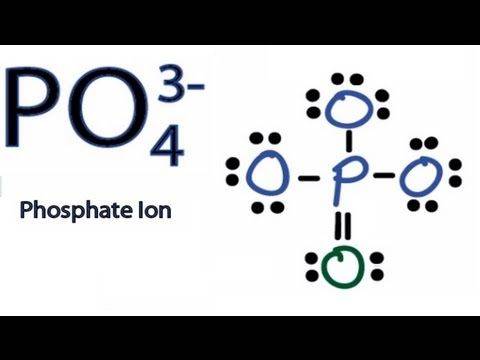
I Understand the 4 represents how many oxygen atoms are bonded to the Phosphate. What exactly does the 3 and its - sign represent? By the image, I can tell the difference between the 3 oxygen atoms and the single isolated one, with the double || symbol, has a different electronic composition. Explain why the single | symbol is used and why the isolated O atom uses two ||s. Please also describe the difference between the two electronic compositions. Why does it show 3 pairs of dots on the O33-s and a double pair on the single O2-