- #1
nonequilibrium
- 1,439
- 2
I've been trying to grasp it very thoroughly (excuse me for my english, I'm belgian) as I have a course of thermodynamics now in my first year of Physics. There is one last thing bothering me about entropy:
Take the Otto-cycle (two adiabats, two ischores/isometrics). We know the cycle is even theoretically irreversible, because its efficiency (which we can calculate) is smaller than Carnot's and all reversible processes have the same efficiency. But now I want to calculate the change of entropy in the universe after one cycle to SEE its irreversibility.
I assume in some way it HAS to be possible to calculate it, because you already know the change of entropy is greater than zero (cf. last paragraph). Take an isochore (I will continue with the isochore from cold to hot [2 -> 3 in bottom image]). My teacher said, after inquiry, that you can calculate the entropy change in the universe (of this isochoric process) by assuming the process is equivalent to placing an infinite reservoir at Th in contact with the gas in a solid container, initially at Tc, until it's warmed up to Th. In this case (not really important how) it can be shown the total entropy increase of the universe is Cv(ln(Th/Tc) + Tc/Th - 1) which can be proven to be greater than zero.
BUT this process is not quasi-static and since you've drawn the cycle in a PV-diagram (indicating at each moment its T,P,V is well-defined), it has to be quasi-static (and ANY non-quasistatic process is irreversible, so it's kind of a lame and non-interesting choice in this case). So a process that would fit would be one where you first place against the gas an infinite reservoir at the temperature of Tc + delta, then Tc + 2delta, ... Th. But in this case the entropy change of the system (which is the same as the earlier case is) is Cvln(Th/Tc) and that of the reservoir/exterior/... is Cvln(Tc/Th) and thus total entropy change zero...
But the Otto cyclus is irreversible
What is the deal?
Thank you tremendously for any help, I've been breaking my head on it,
Ruben
PS: if my explenation was hard to follow, feel free to ignore it, basically my question is: how can I calculate the change in the universal entropy (i.e.: show it is irreversible) after going through one isochoric process in an Otto-cycle
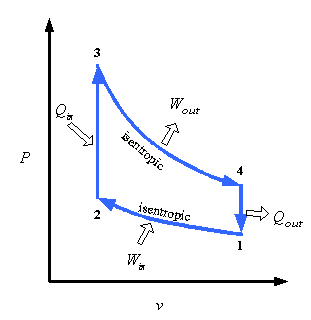
Take the Otto-cycle (two adiabats, two ischores/isometrics). We know the cycle is even theoretically irreversible, because its efficiency (which we can calculate) is smaller than Carnot's and all reversible processes have the same efficiency. But now I want to calculate the change of entropy in the universe after one cycle to SEE its irreversibility.
I assume in some way it HAS to be possible to calculate it, because you already know the change of entropy is greater than zero (cf. last paragraph). Take an isochore (I will continue with the isochore from cold to hot [2 -> 3 in bottom image]). My teacher said, after inquiry, that you can calculate the entropy change in the universe (of this isochoric process) by assuming the process is equivalent to placing an infinite reservoir at Th in contact with the gas in a solid container, initially at Tc, until it's warmed up to Th. In this case (not really important how) it can be shown the total entropy increase of the universe is Cv(ln(Th/Tc) + Tc/Th - 1) which can be proven to be greater than zero.
BUT this process is not quasi-static and since you've drawn the cycle in a PV-diagram (indicating at each moment its T,P,V is well-defined), it has to be quasi-static (and ANY non-quasistatic process is irreversible, so it's kind of a lame and non-interesting choice in this case). So a process that would fit would be one where you first place against the gas an infinite reservoir at the temperature of Tc + delta, then Tc + 2delta, ... Th. But in this case the entropy change of the system (which is the same as the earlier case is) is Cvln(Th/Tc) and that of the reservoir/exterior/... is Cvln(Tc/Th) and thus total entropy change zero...
But the Otto cyclus is irreversible
What is the deal?
Thank you tremendously for any help, I've been breaking my head on it,
Ruben
PS: if my explenation was hard to follow, feel free to ignore it, basically my question is: how can I calculate the change in the universal entropy (i.e.: show it is irreversible) after going through one isochoric process in an Otto-cycle
Last edited: