- #1
DespicableMe
- 41
- 0
For the elimination reaction of ethanol:
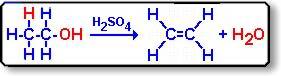
How come only the OH and H get taken out? I understand that you have to take out one atom from each carbon, so how come we can't take out, for example, one H from the left carbon and one H from the right carbon to get?
Or is it mandatory to take out the hydroxyl from an alcohol?
How come only the OH and H get taken out? I understand that you have to take out one atom from each carbon, so how come we can't take out, for example, one H from the left carbon and one H from the right carbon to get?
Or is it mandatory to take out the hydroxyl from an alcohol?
Last edited: