- #1
vis viva
- 29
- 10
- TL;DR Summary
- What happens in the boundary area between Cu(s) and Cu2+(a)? How does the various particles respond to each others presence?
Please behold my newest graphical masterpiece here below. The winner of this expensive artwork will be the first that can help me understand how the various particles respond to each others presence?
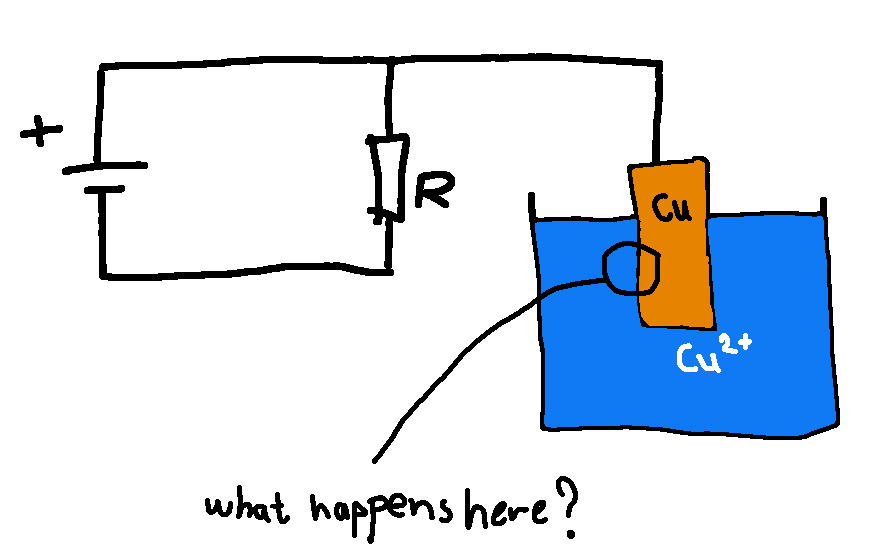
The Cu2+ is supposed to depict CuSO4 pentahydrate in water, so I guess the correct term would be [Cu(H2O)6]2+ if we are going to nitpick.
The Cu2+ is supposed to depict CuSO4 pentahydrate in water, so I guess the correct term would be [Cu(H2O)6]2+ if we are going to nitpick.