- #1
lalbatros
- 1,256
- 2
Hello,
Here is the Boltzmann collision term as it is expressed on http://en.wikipedia.org/wiki/Boltzmann_equation" :
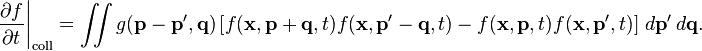
Deriving the H theorem from this equation is the way to usually prove of the irreversibility of this term.
Of course, this collision term should not be symmetric by time reversal.
I guess it is easy to prove that without recourse to the H theorem.
How could I do (see) that?
Thanks,
Michel
Here is the Boltzmann collision term as it is expressed on http://en.wikipedia.org/wiki/Boltzmann_equation" :
Deriving the H theorem from this equation is the way to usually prove of the irreversibility of this term.
Of course, this collision term should not be symmetric by time reversal.
I guess it is easy to prove that without recourse to the H theorem.
How could I do (see) that?
Thanks,
Michel
Last edited by a moderator: