- #1
Stephanus
- 1,316
- 104
Dear PF Forum,
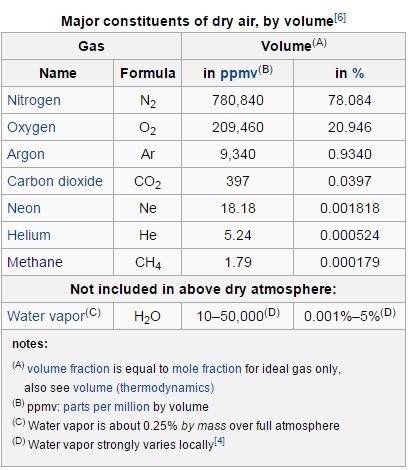
Our atmosphere consist of 78% N2, 21% O2, 0.9% Argon, and other...
https://en.wikipedia.org/wiki/Atmosphere_of_Earth
What about theses gases? Will they form a layer like this liquid because of their buoyancy difference?

Or they will be scattered evenly because of the wind.
I think they will be scattered evenly. But I'm just curious.
Our atmosphere consist of 78% N2, 21% O2, 0.9% Argon, and other...
https://en.wikipedia.org/wiki/Atmosphere_of_Earth
What about theses gases? Will they form a layer like this liquid because of their buoyancy difference?
Or they will be scattered evenly because of the wind.
I think they will be scattered evenly. But I'm just curious.