- #1
rednass
Hello,
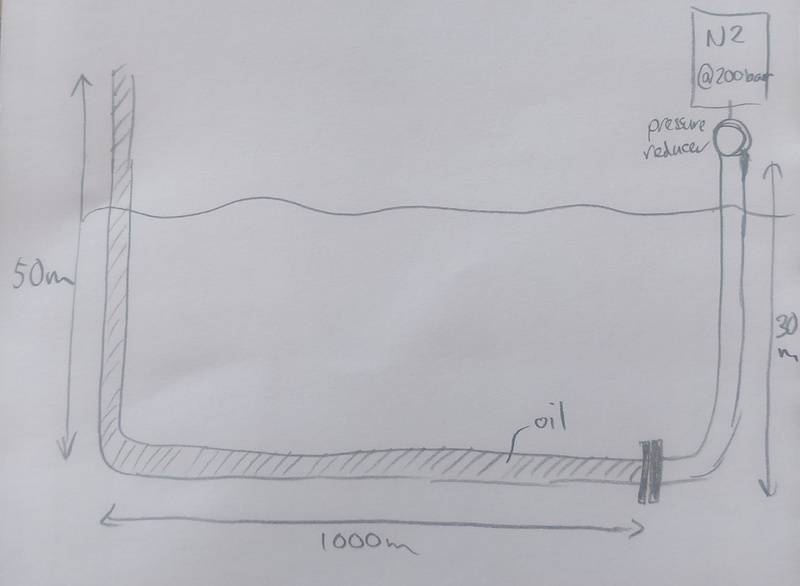
I am trying to solve a problem where I want to know how many cylinders of N2 (50L, 200 bar) are needed to flush a pipe filled with oil.
The pipe diameter is 0.5", so the volume of the pipe is 0.133 m3 (with a length of 1050m) which is filled with oil.
Behind the N2 cylinders is a pressure reducer which can be set from 0-10 bar.
How can I calculate the number of cylinders I need of N2?
I need to overcome the height of 50m + 1 bar, so around 6 bar. Let's say I set the pressure reducer to 10 bar and flush the pipe, then it contains 0.133 m3 @ 10 bar = 1.33 m3 @ 1 bar.
If, let's say the last 15 bar can't be taken out of the cylinder, it contains effectively 9250 L @ 1 bar = 9.25 m3 @ 1 bar.
Can I just compare those two and say 9.25 > 1.33, so only 1 cylinder is enough?
Thanks in advance!
I am trying to solve a problem where I want to know how many cylinders of N2 (50L, 200 bar) are needed to flush a pipe filled with oil.
The pipe diameter is 0.5", so the volume of the pipe is 0.133 m3 (with a length of 1050m) which is filled with oil.
Behind the N2 cylinders is a pressure reducer which can be set from 0-10 bar.
How can I calculate the number of cylinders I need of N2?
I need to overcome the height of 50m + 1 bar, so around 6 bar. Let's say I set the pressure reducer to 10 bar and flush the pipe, then it contains 0.133 m3 @ 10 bar = 1.33 m3 @ 1 bar.
If, let's say the last 15 bar can't be taken out of the cylinder, it contains effectively 9250 L @ 1 bar = 9.25 m3 @ 1 bar.
Can I just compare those two and say 9.25 > 1.33, so only 1 cylinder is enough?
Thanks in advance!