- #1
Dopplerganger
- 2
- 0
Hi all,
I'm trying to come up with velocity equations for a multi-enzymatic system and came across this velocity equation. Does anyone recognize this equation?
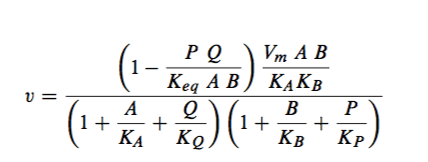
There was no citation or derivation and the variables were not explained. This is my guess:
I'm trying to come up with velocity equations for a multi-enzymatic system and came across this velocity equation. Does anyone recognize this equation?
There was no citation or derivation and the variables were not explained. This is my guess:
- P, Q, A, B are concentrations of some sort
- Keq is the equilibrium constant
- Ka, Kb, Kp, Kq are the Michaelis constants