- #1
iakmngle
- 3
- 0
- Homework Statement
- Give an expression for the number of states, N , having energy less than some given E .
Assume N >> 1.
- Relevant Equations
- $$
\psi (x,y,z) \equiv A
\sin{\left(\frac{n_x \pi x}{a} \right)}
\sin{\left(\frac{n_y \pi y}{a} \right)}
\sin{\left(\frac{n_z \pi z}{a} \right)}
$$
Hi, so I'm having trouble with a homework problem where it asks me to find the number of states with an energy less than some given E.
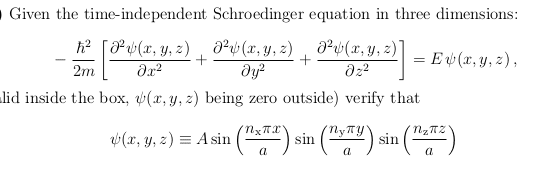
From this, I was able to work out the energy E to be
$$
E = \frac{\hbar^2}{2m} \frac{\pi^2}{a^2} \left(
n_x^2 + n_y^2 + n_z^2
\right)
$$
and also find the energy of the ground and first excited states respectively by replacing (nx,ny,nz) with (1,1,1) and (1,1,2).
I've attempted to rearrange the equation as below, but am not really sure about where to go next.
$$
n_x^2 + n_y^2 + n_z^2 = \frac{2m E a^2}{\hbar^2 \pi^2}
$$
Any guidance would be appreciated. Thanks in advance!
From this, I was able to work out the energy E to be
$$
E = \frac{\hbar^2}{2m} \frac{\pi^2}{a^2} \left(
n_x^2 + n_y^2 + n_z^2
\right)
$$
and also find the energy of the ground and first excited states respectively by replacing (nx,ny,nz) with (1,1,1) and (1,1,2).
I've attempted to rearrange the equation as below, but am not really sure about where to go next.
$$
n_x^2 + n_y^2 + n_z^2 = \frac{2m E a^2}{\hbar^2 \pi^2}
$$
Any guidance would be appreciated. Thanks in advance!
Last edited: