- #1
athosanian
- 67
- 8
hi, in perovskite crystal, such as BaTiO3, due to the off-centre of Ti ion, there exists a polarization moment in the unit cell (as shown in below link). my question is: does the disposition of Ti atom lead to separation of electron cloud from the nuclei of the Ti atom and so the Ti atom plays as a electric dipole ?
https://www.comsol.com/blogs/piezoelectric-materials-crystal-orientation-poling-direction/
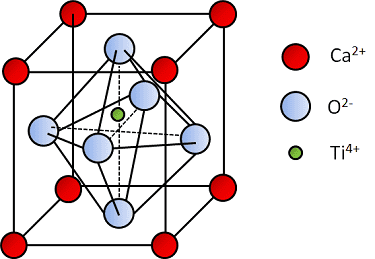
https://www.comsol.com/blogs/piezoelectric-materials-crystal-orientation-poling-direction/