- #1
Phys12
- 351
- 42
So I just learned that in a spectrum like this one:
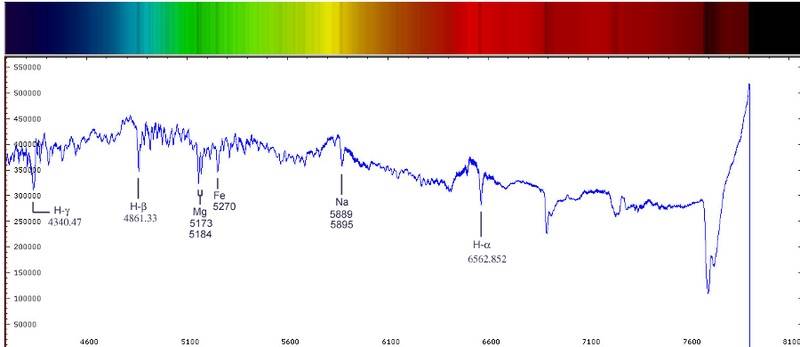
(For now, let's concentrate on the graph given below.)
The longer the dip is in the graph, the more the corresponding element is present. However, if each individual color corresponds to a different element, then how can a larger dip mean more of that element. As far as I know, when we take a spectra, we usually get something as it's given in the figure above the graph (the rainbow colored spectra) and I observe that deeper the dip is, more is the blackening in that colored spectrum. But how do we really know that the dip really corresponds to the same element?
If we get a spectrum which is completely black for 1 cm. around Mg and Fe. This would show in the graph as a big dip which stays for a long time. Then what can we deduce about the object we're looking at? Does it have both Mg and Fe a lot or does it have a lot of Mg?
(For now, let's concentrate on the graph given below.)
The longer the dip is in the graph, the more the corresponding element is present. However, if each individual color corresponds to a different element, then how can a larger dip mean more of that element. As far as I know, when we take a spectra, we usually get something as it's given in the figure above the graph (the rainbow colored spectra) and I observe that deeper the dip is, more is the blackening in that colored spectrum. But how do we really know that the dip really corresponds to the same element?
If we get a spectrum which is completely black for 1 cm. around Mg and Fe. This would show in the graph as a big dip which stays for a long time. Then what can we deduce about the object we're looking at? Does it have both Mg and Fe a lot or does it have a lot of Mg?