- #1
Douasing
- 41
- 0
For violet phophorus,it seems that the crystal structures given by two websites are not the same.In Openstax CNX,the link is http://cnx.org/contents/f46e8679-ee00-4073-9f5e-a87ca9955a9e@25.9:67/Chemistry_of_the_Main_Group_El
and the Figure 12 displays it as follows:
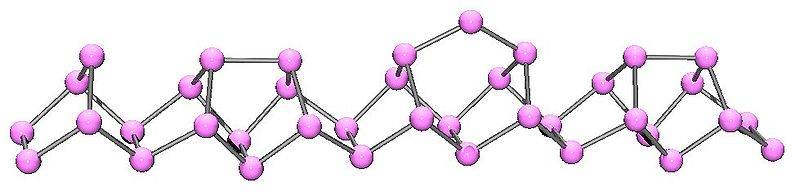
But in Chemwiki,the link is http://chemwiki.ucdavis.edu/Inorgan..._The_Nitrogen_Family/Chemistry_of_Phosphorous
and the Figure 3 displays it as follows:
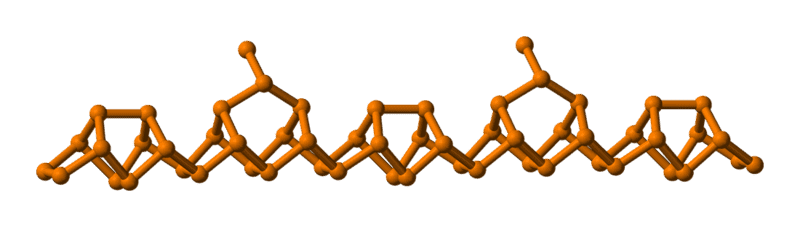
Which is right for these two forms of structure ?
There is a double bond between two phosphorus in the first figure,is the structure reseanable?
There is a dangling bond in the phosphorus in the second one, is the structure stable ?
---------------------------------------------------------------------------------------
For red phosphorus, the Wikipedia does not show a clear figure,for example
http://en.wikipedia.org/wiki/Allotropes_of_phosphorus
http://en.wikipedia.org/wiki/Phosphorus#cite_note-berger-8
who can supply a clear figure of the crystal structure?
and the Figure 12 displays it as follows:
But in Chemwiki,the link is http://chemwiki.ucdavis.edu/Inorgan..._The_Nitrogen_Family/Chemistry_of_Phosphorous
and the Figure 3 displays it as follows:
Which is right for these two forms of structure ?
There is a double bond between two phosphorus in the first figure,is the structure reseanable?
There is a dangling bond in the phosphorus in the second one, is the structure stable ?
---------------------------------------------------------------------------------------
For red phosphorus, the Wikipedia does not show a clear figure,for example
http://en.wikipedia.org/wiki/Allotropes_of_phosphorus
http://en.wikipedia.org/wiki/Phosphorus#cite_note-berger-8
who can supply a clear figure of the crystal structure?