- #1
dweeegs
- 12
- 1
I'm trying to model a Transfer function for the response of the deviation in temperature of water coming out of the concentric tube heat exchanger in response to an input velocity of water function.
THE PROBLEM
I start the derivation with a couple equations in Seborg's Process Dynamics and Controls and then go off on deriving on my own since my application of the equations are different then what they were using them to show.
A short snippet before I started my derivation:
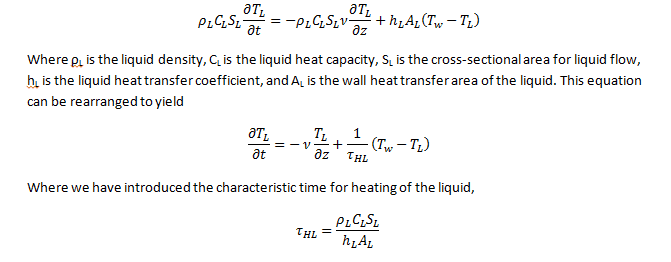
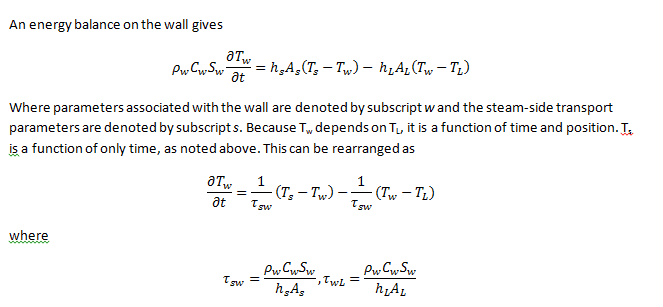
Now I understand what A_s and A_L are (the area available for heat transfer for those two... the length times 2(pi)R for the outside and inside radii, respectively).
What the heck is Aw? Is that an average of the two areas or the total amount of area available for heat transfer?
I got my model into standard form for what I want, but I'm having trouble putting in actual numbers.
Any help is much appreciated!
THE PROBLEM
I start the derivation with a couple equations in Seborg's Process Dynamics and Controls and then go off on deriving on my own since my application of the equations are different then what they were using them to show.
A short snippet before I started my derivation:
Now I understand what A_s and A_L are (the area available for heat transfer for those two... the length times 2(pi)R for the outside and inside radii, respectively).
What the heck is Aw? Is that an average of the two areas or the total amount of area available for heat transfer?
I got my model into standard form for what I want, but I'm having trouble putting in actual numbers.
Any help is much appreciated!