- #1
Janiceleong26
- 276
- 4
1. Homework Statement
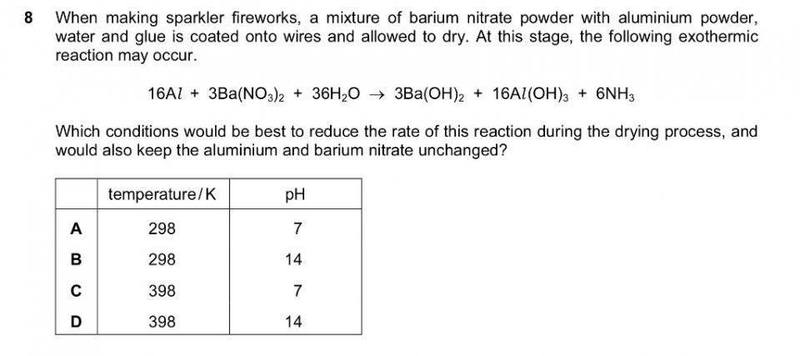
I chose B, because I thought adding more OH- would cause the reaction to shift to the left, and a low temperature would slow the rate of reaction, but the answer is A, why pH 7?
Homework Equations
The Attempt at a Solution
I chose B, because I thought adding more OH- would cause the reaction to shift to the left, and a low temperature would slow the rate of reaction, but the answer is A, why pH 7?